30) What is the percent yield for the reaction below? Only 28.16 g of CO2 are observed to have formed even though stoichiometry would predict the production of 225.3 g of CO2 since 4.000 (l00 moles of C8H18 and 8.000 moles of O2 were used. 2 C3H18 + 25 O2 16 CO2 + 18 H2O A) 12.50% B) 25.00% C) 50.00% 20.00%
30) What is the percent yield for the reaction below? Only 28.16 g of CO2 are observed to have formed even though stoichiometry would predict the production of 225.3 g of CO2 since 4.000 (l00 moles of C8H18 and 8.000 moles of O2 were used. 2 C3H18 + 25 O2 16 CO2 + 18 H2O A) 12.50% B) 25.00% C) 50.00% 20.00%
Chemistry: The Molecular Science
5th Edition
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:John W. Moore, Conrad L. Stanitski
Chapter3: Chemical Reactions
Section3.8: Evaluating Chemical Synthesis: Percent Yield
Problem 3.18PSP: Assume the methanol synthesis has an 85.0% yield and you want to make 1.00 kg CH3OH. Calculate the...
Related questions
Question
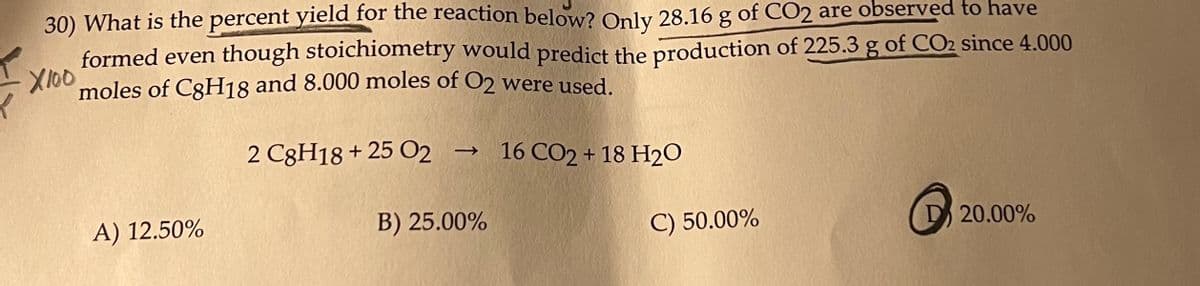
Transcribed Image Text:30) What is the percent yield for the reaction below? Only 28.16 g of CO2 are observed to have
formed even though stoichiometry would predict the production of 225.3 g of CO2 since 4.000
X100 moles of C3H18 and 8.000 moles of O2 were used.
2 C8H18+ 25 O2 → 16 CO2 + 18 H2O
A) 12.50%
B) 25.00%
C) 50.00%
20.00%
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning