Nitric acid is a key industrial chemical, largely used to make fertilizers and explosives. The first step in its synthesis is the oxidation of ammonia. In this reaction, gaseous ammonia reacts with dioxygen gas to produce nitrogen monoxide gas and water. Suppose a chemical engineer studying a new catalyst for the oxidation of ammonia reaction finds that 358. liters per second of dioxygen are consumed when the reaction is run at 287. °C and the dioxygen is supplied at 0.13 atm. Calculate the rate at which nitrogen monoxide is being produced. Give your answer in kilograms per second. Round your answer to 2 significant digits. kg
Nitric acid is a key industrial chemical, largely used to make fertilizers and explosives. The first step in its synthesis is the oxidation of ammonia. In this reaction, gaseous ammonia reacts with dioxygen gas to produce nitrogen monoxide gas and water. Suppose a chemical engineer studying a new catalyst for the oxidation of ammonia reaction finds that 358. liters per second of dioxygen are consumed when the reaction is run at 287. °C and the dioxygen is supplied at 0.13 atm. Calculate the rate at which nitrogen monoxide is being produced. Give your answer in kilograms per second. Round your answer to 2 significant digits. kg
Chapter5: Gases
Section: Chapter Questions
Problem 74E: Urea (H2NCONH2) is used extensively as a nitrogen source in fertilizers. It is produced commercially...
Related questions
Question
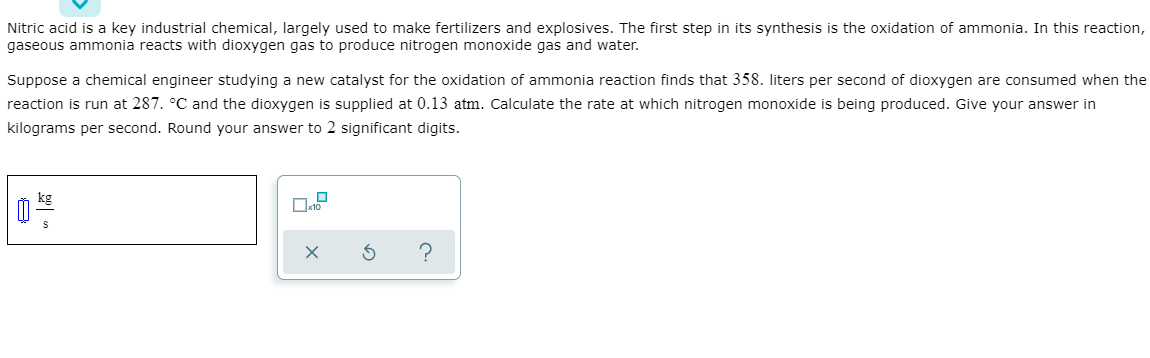
Transcribed Image Text:Nitric acid is a key industrial chemical, largely used to make fertilizers and explosives. The first step in its synthesis is the oxidation of ammonia. In this reaction,
gaseous ammonia reacts with dioxygen gas to produce nitrogen monoxide gas and water.
Suppose a chemical engineer studying a new catalyst for the oxidation of ammonia reaction finds that 358. liters per second of dioxygen are consumed when the
reaction is run at 287. °C and the dioxygen is supplied at 0.13 atm. Calculate the rate at which nitrogen monoxide is being produced. Give your answer in
kilograms per second. Round your answer to 2 significant digits.
kg
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps

Recommended textbooks for you


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781285199030
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning