nitric acid is made by oxidation of nh3. 3 reactions occur. below are the following rxns. (a) plz balance them... (b) use a generation consumption analysis to synthesize a reaction pathway to make nitric acid from NH3 & 02 based on these 3 rxns, with NO net generation or consumption of N0 and NO3. h20 is allowed.
nitric acid is made by oxidation of nh3. 3 reactions occur. below are the following rxns. (a) plz balance them... (b) use a generation consumption analysis to synthesize a reaction pathway to make nitric acid from NH3 & 02 based on these 3 rxns, with NO net generation or consumption of N0 and NO3. h20 is allowed.
Chapter7: Statistical Data Treatment And Evaluation
Section: Chapter Questions
Problem 7.9QAP
Related questions
Question
nitric acid is made by oxidation of nh3. 3 reactions occur. below are the following rxns.
(a) plz balance them...
(b) use a generation consumption analysis to synthesize a reaction pathway to make nitric acid from NH3 & 02 based on these 3 rxns, with NO net generation or consumption of N0 and NO3. h20 is allowed.
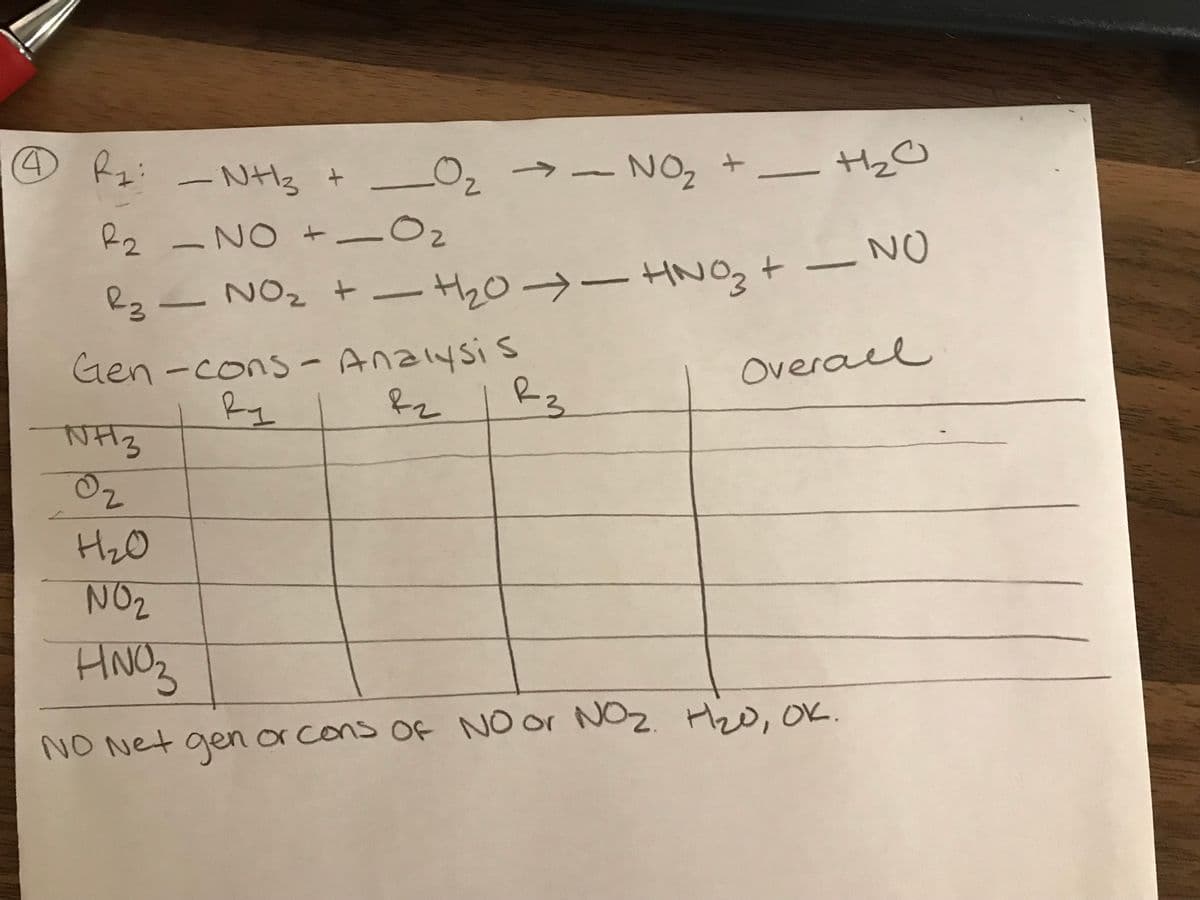
Transcribed Image Text:-NH3 + _O2
NO, + HO
->
-NO +-Oz
02
HNO3
23ー N02 + ーtho→-tzt ー N0
Gen-cons - Ana1ysis
Rz
Overael
Ry
NH3
NO2
HNC
NO Net gen or cons of NO or NO2. Hzo, oK.
2.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps

Recommended textbooks for you


Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning


Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning