Nitrogen ga Nhan asolubility in water of appronimately 0.0215et at 250Cand 0.99 atm What is the solubility la/of Nyinwater in Denver, wherethe atmopheric pressure is approximately0.689 atm Do not report units in your answer Report your answer with 4 places past the decimal point
Nitrogen ga Nhan asolubility in water of appronimately 0.0215et at 250Cand 0.99 atm What is the solubility la/of Nyinwater in Denver, wherethe atmopheric pressure is approximately0.689 atm Do not report units in your answer Report your answer with 4 places past the decimal point
Chemistry: Matter and Change
1st Edition
ISBN:9780078746376
Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Chapter14: Mixtures And Solutions
Section: Chapter Questions
Problem 94A
Related questions
Question

Transcribed Image Text:Nitrogen gas (Nal has a solubility in water of appronimately 0.0215et at 25.0C and 0.99 atm What is the solubility le/ of Ny in water in Denver, where the atmonheri
pressure is approximately 0.889 atm
Do not report units in your answer Report your answer with 4 places past the decimal point
Type your answer
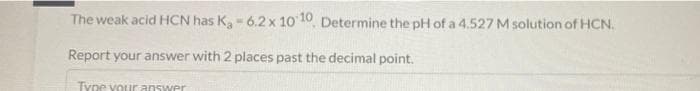
Transcribed Image Text:The weak acid HCN has K,- 6.2x 10 10, Determine the pH of a 4.527 M solution of HCN.
Report your answer with 2 places past the decimal point.
Type vour answer
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co

Chemistry for Today: General, Organic, and Bioche…
Chemistry
ISBN:
9781305960060
Author:
Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co

Chemistry for Today: General, Organic, and Bioche…
Chemistry
ISBN:
9781305960060
Author:
Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning