Nitroglycerin (C3H5N309 ) is a powerful explosive. Its decomposition may be represented by 4C3H5N309 → 6N2 + 12CO2 + 10 H20 + 02 This reaction generates a large amount of heat and many gaseous products. It is the sudden formation of these gases, together with their rapid expansion, that produces the explosion. (a) What is the maximum amount of O2 in grams that can be obtained from 2.00 x 10² g of nitroglycerin? (b) Calculate the percent yield in this reaction if the amount of O2 generated is found to be 6.55 g.
Nitroglycerin (C3H5N309 ) is a powerful explosive. Its decomposition may be represented by 4C3H5N309 → 6N2 + 12CO2 + 10 H20 + 02 This reaction generates a large amount of heat and many gaseous products. It is the sudden formation of these gases, together with their rapid expansion, that produces the explosion. (a) What is the maximum amount of O2 in grams that can be obtained from 2.00 x 10² g of nitroglycerin? (b) Calculate the percent yield in this reaction if the amount of O2 generated is found to be 6.55 g.
Chemistry: The Molecular Science
5th Edition
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:John W. Moore, Conrad L. Stanitski
Chapter8: Properties Of Gases
Section: Chapter Questions
Problem 129QRT
Related questions
Question
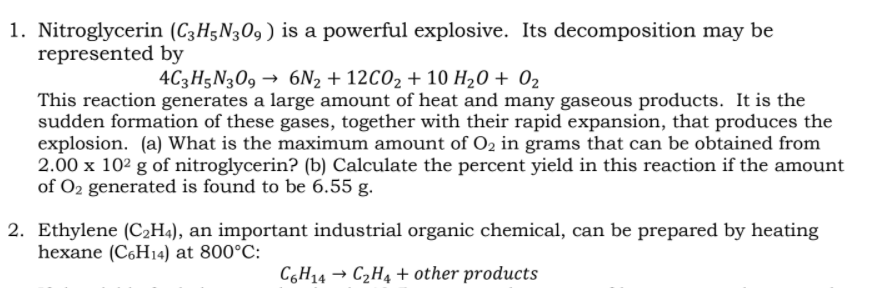
Transcribed Image Text:1. Nitroglycerin (C3H5N309 ) is a powerful explosive. Its decomposition may be
represented by
4C3H5N309 → 6N2 + 12CO2 + 10 H20 + 02
This reaction generates a large amount of heat and many gaseous products. It is the
sudden formation of these gases, together with their rapid expansion, that produces the
explosion. (a) What is the maximum amount of O2 in grams that can be obtained from
2.00 x 102 g of nitroglycerin? (b) Calculate the percent yield in this reaction if the amount
of O2 generated is found to be 6.55 g.
2. Ethylene (C2H«), an important industrial organic chemical, can be prepared by heating
hexane (C6H14) at 800°C:
C6H14 → C2H4 + other products

Transcribed Image Text:Direction: Analyzed and solve each problem given on each item. Write your calculation on a
separate sheet of paper.
Note: In calculation involving atomic mass, use 4 significant figure for the atomic mass of each
element needed in the calculation. Use scientific notation, if necessary.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 5 steps

Recommended textbooks for you

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning