no subject-fantil Oudeledux Mal-Francesca A Tantillo-Out x → C app.101edu.co < D A Q 1 ! Z 95°F Mostly sunny o 2 W S X Solid 4x 200 3 E D Liquid Gas Temperature (K) 4 $ 4 R F V START HERE-CHM150-251 Chen X SCF O % 5 T G A phase diagram for CO₂ is shown below. R A At STP, CO₂ will exist as a 6 Aktiv Chemistry Y Question 21 of 37 H * & 7 U J PrtScn * 8 A) solid B) liquid C) gas D) supercritical fluid (SCF) 1 0 Home K ( 9 N O End F10 L ) 0 PgUp P PgDn Fiz + 4 91 0 Update Submit 1:27 PM 7/23/2022 Backspace Ent
no subject-fantil Oudeledux Mal-Francesca A Tantillo-Out x → C app.101edu.co < D A Q 1 ! Z 95°F Mostly sunny o 2 W S X Solid 4x 200 3 E D Liquid Gas Temperature (K) 4 $ 4 R F V START HERE-CHM150-251 Chen X SCF O % 5 T G A phase diagram for CO₂ is shown below. R A At STP, CO₂ will exist as a 6 Aktiv Chemistry Y Question 21 of 37 H * & 7 U J PrtScn * 8 A) solid B) liquid C) gas D) supercritical fluid (SCF) 1 0 Home K ( 9 N O End F10 L ) 0 PgUp P PgDn Fiz + 4 91 0 Update Submit 1:27 PM 7/23/2022 Backspace Ent
Chemistry: Principles and Reactions
8th Edition
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:William L. Masterton, Cecile N. Hurley
Chapter1: Matter And Measurements
Section: Chapter Questions
Problem 16QAP: Computers are not supposed to be in very warm rooms. The highest temperature tolerated for maximum...
Related questions
Question
Transcribed Image Text:D
(no subject-fantilOudeledux Mail-Francesca A Tantillo-Out x
- C app.101edu.co
<
A
1
Q
Z
C
1
95°F
Mostly sunny
FT
2
@
W
S
X
Alt
Solid
4x
200
#
3
E
D
Liquid
Gas
Temperature (K)
4
$
4
R
HO
F
V
START HERE-CHM150-251 Chen X
SCF
%
5
T
G
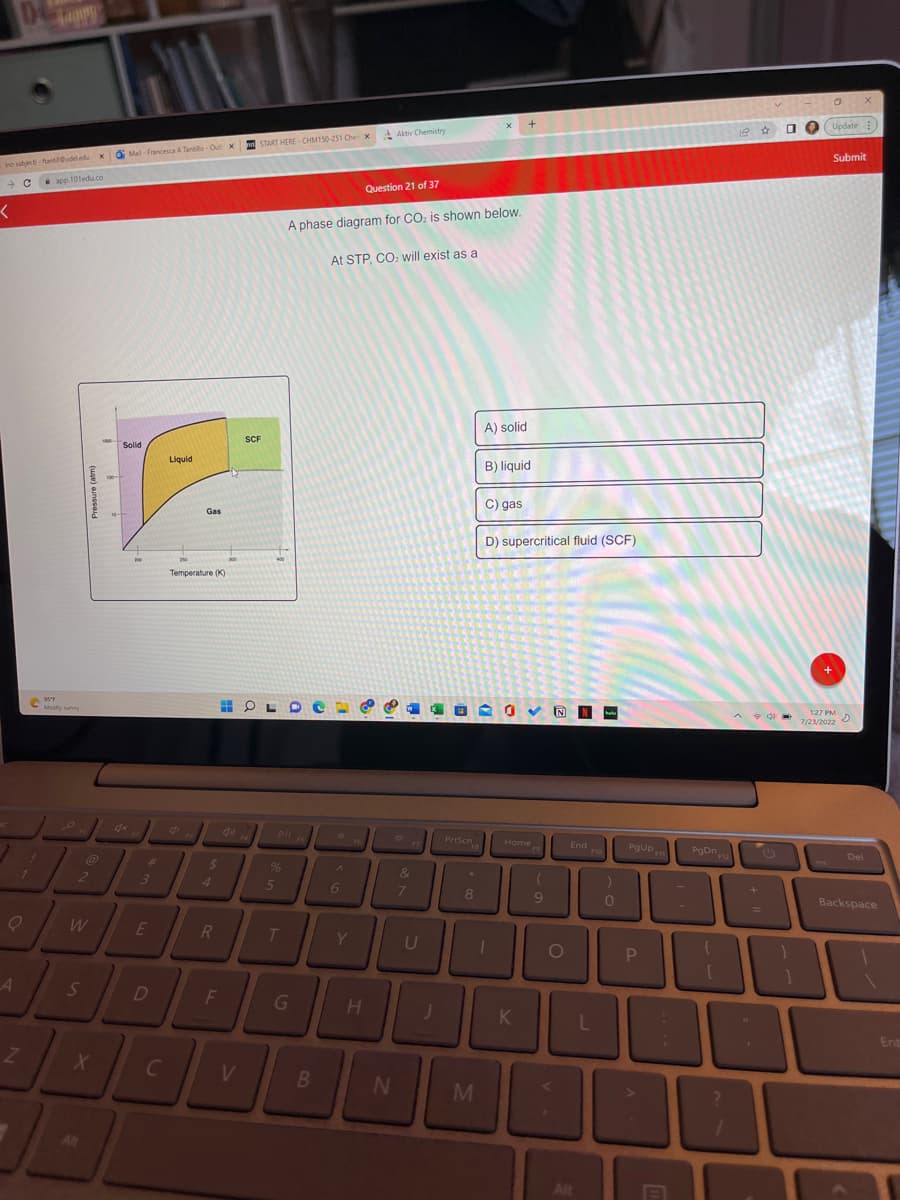
A phase diagram for CO₂ is shown below.
B
A
At STP, CO₂ will exist as a
6
Y
Aktiv Chemistry
Question 21 of 37
H
30
N
&
7
U
J
PrtScn
*
8
M
A) solid
B) liquid
C) gas
D) supercritical fluid (SCF)
0
Home
K
(
9
N
O
v
End
F10
L
)
0
PgUp
P
E
PgDn Fiz
1.4
+
4
91
0
Update
Submit
1:27 PM
7/23/2022
Backspace
Ent
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning


Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning
