Chemistry & Chemical Reactivity
9th Edition
ISBN:9781133949640
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Chapter24: Biochemistry
Section24.3: Nucleic Acids
Problem 1RC: 1. Which breaks down more quickly in an aqueous solution?
DNA
RNA
Related questions
Question
Complete the synthesis reaction showing curved arrows and mechanisms for each reaction.
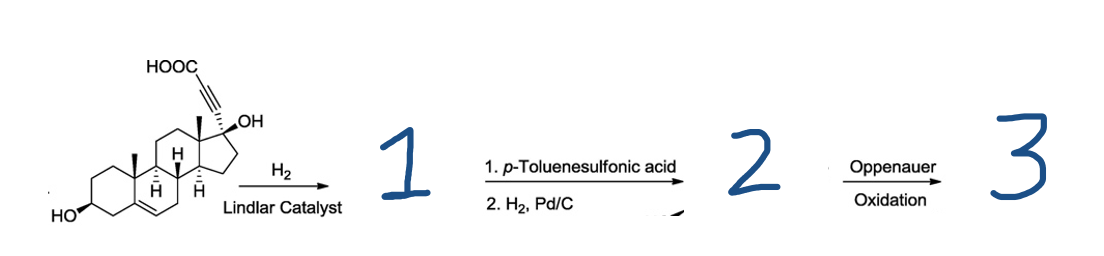
Transcribed Image Text:НООС
HO
2.
H2
1. p-Toluenesulfonic acid
Oppenauer
Lindlar Catalyst
2. Н2, Pа/c
Oxidation
HO
Expert Solution
Step 1
For the above organic synthesis, the following transformations must be known:
1. H2 in presence of Lindlar's catalyst reduces alkyne to cis-alkene.
2. p-Toluenesulfonic acid reacts with 3o alcohol to form tosylate and elimination occurs.
3. H2 , Pd/C reduces alkenes to alkanes.
4. Oppenauer Oxidation is a simple method for selectively oxidizing 2o acohols to ketones.
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning