nswer: ne oxidation number of oxygen in OF2 is -2. elect one: O True O False 12 M Solution of hydrochloric acid of volume 6 L needed to be dilu /hat is the volume of the solution after dilution. О а. 24 L O b. 12 L О с. 18 L O d. 16 L
nswer: ne oxidation number of oxygen in OF2 is -2. elect one: O True O False 12 M Solution of hydrochloric acid of volume 6 L needed to be dilu /hat is the volume of the solution after dilution. О а. 24 L O b. 12 L О с. 18 L O d. 16 L
Chapter18: Electrochemistry
Section: Chapter Questions
Problem 115AE: The saturated calomel electrode. abbreviated SCE. is often used as a reference electrode in making...
Related questions
Question
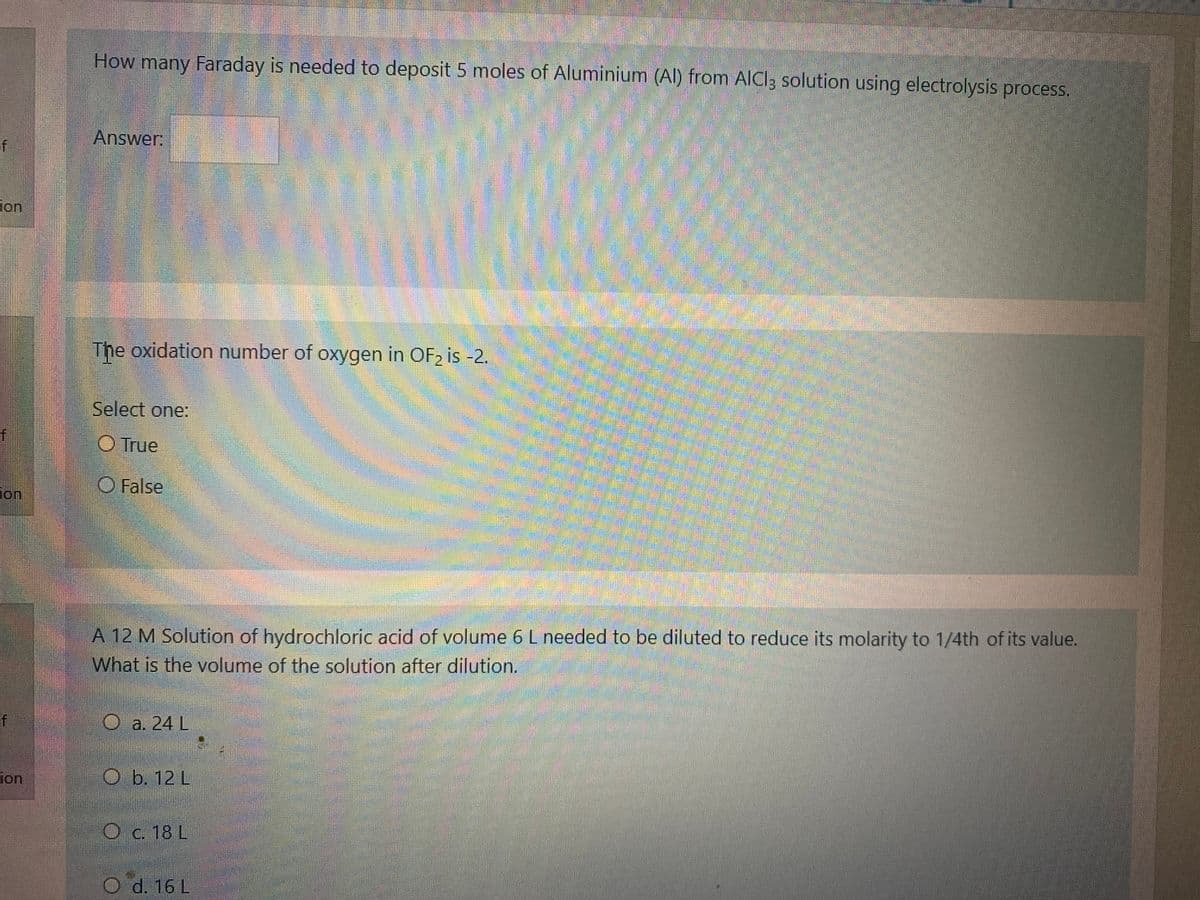
Transcribed Image Text:How many Faraday is needed to deposit 5 moles of Aluminium (Al) from AICl3 solution using electrolysis process.
Answer:
of
ion
The oxidation number of oxygen in OF2 is -2.
Select one:
f
O True
O False
Jon
A 12 M Solution of hydrochloric acid of volume 6 L needed to be diluted to reduce its molarity to 1/4th of its value.
What is the volume of the solution after dilution.
of
O a. 24 L
ion
O b. 12 L
O c. 18 L
d. 16 L
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax