Nucleophilic aromatic substitution involves the formation of a resonance-stabilized carbanion intermediate called a Meisenheimer complex as the nucleophile attacks the ring carbon carrying the eventual leaving group; electron-withdrawing groups ortho and/or para to the site of attack help to stabilize this structure via resonance. For the reaction below, draw the structure of the stabilized reaction intermediate in the box below. CI F3C CF3 NO₂ F3C N CF3 NO₂ • You do not have to consider stereochemistry. • Draw the Meisenheimer complex with a formal charge of 0 on the nucleophilic atom. If more than one resonance structure is possible, only draw the most important one.
Nucleophilic aromatic substitution involves the formation of a resonance-stabilized carbanion intermediate called a Meisenheimer complex as the nucleophile attacks the ring carbon carrying the eventual leaving group; electron-withdrawing groups ortho and/or para to the site of attack help to stabilize this structure via resonance. For the reaction below, draw the structure of the stabilized reaction intermediate in the box below. CI F3C CF3 NO₂ F3C N CF3 NO₂ • You do not have to consider stereochemistry. • Draw the Meisenheimer complex with a formal charge of 0 on the nucleophilic atom. If more than one resonance structure is possible, only draw the most important one.
Organic Chemistry
8th Edition
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Chapter18: Functional Derivatives Of Carboxylic Acids
Section: Chapter Questions
Problem 18.70P
Related questions
Question
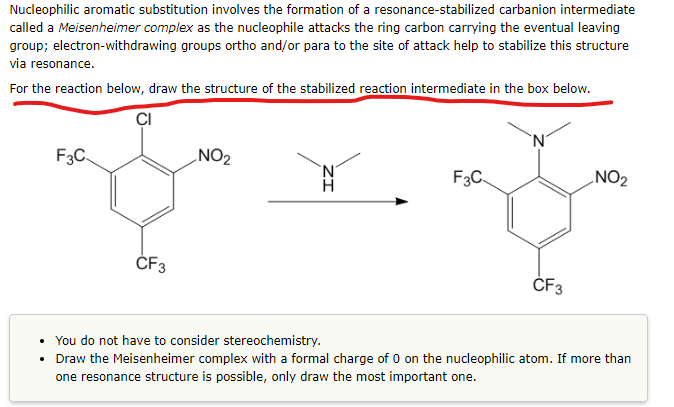
Transcribed Image Text:Nucleophilic aromatic substitution involves the formation of a resonance-stabilized carbanion intermediate
called a Meisenheimer complex as the nucleophile attacks the ring carbon carrying the eventual leaving
group; electron-withdrawing groups ortho and/or para to the site of attack help to stabilize this structure
via resonance.
For the reaction below, draw the structure of the stabilized reaction intermediate in the box below.
CI
F3C
CF3
NO₂
F3C
N
CF3
NO₂
• You do not have to consider stereochemistry.
• Draw the Meisenheimer complex with a formal charge of 0 on the nucleophilic atom. If more than
one resonance structure is possible, only draw the most important one.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning


Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning


EBK A SMALL SCALE APPROACH TO ORGANIC L
Chemistry
ISBN:
9781305446021
Author:
Lampman
Publisher:
CENGAGE LEARNING - CONSIGNMENT