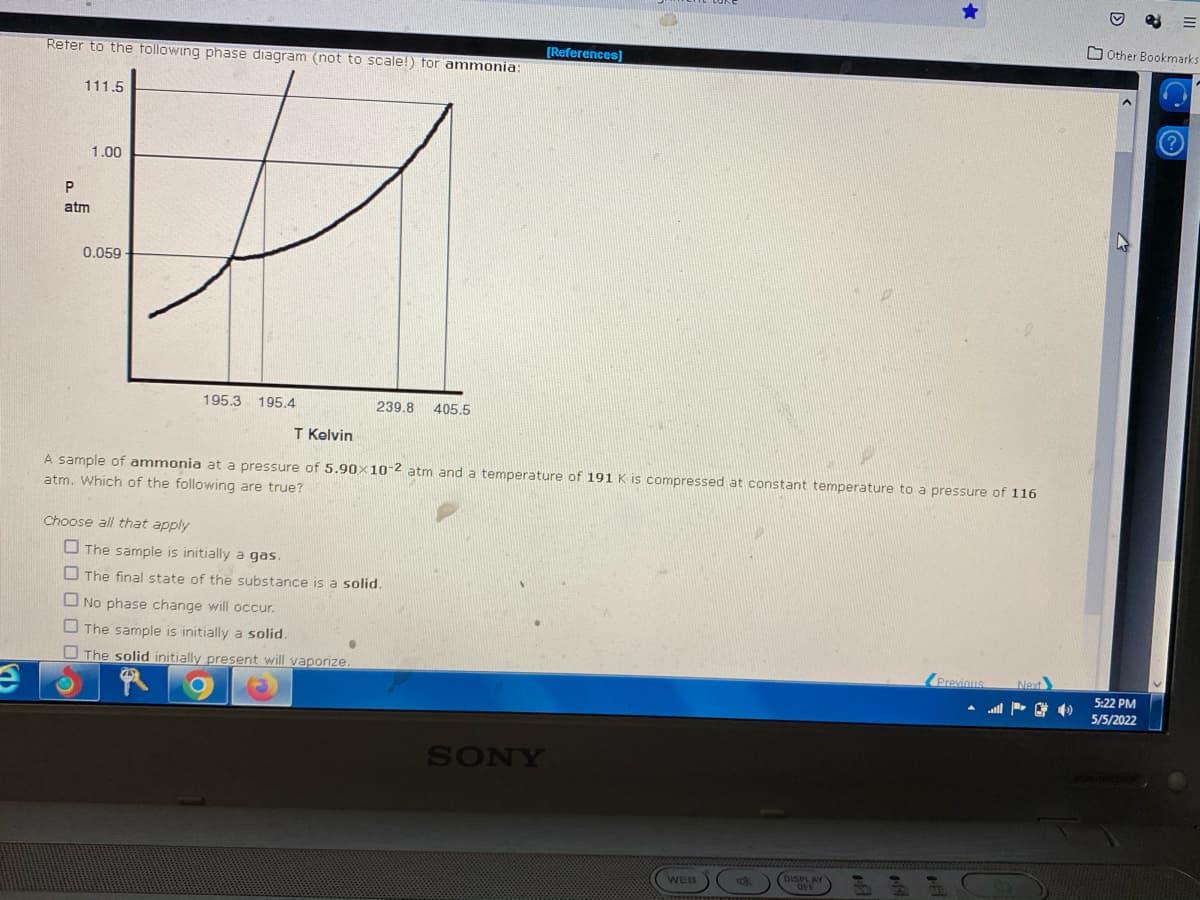
O Oth (References] Reter to the tollowing phase diagram (not to scale!) tor ammonia: 111.5 1.00 atm 0.059 195.3. 195.4 239.8 405.5 T Kolvin A sample of ammonia at a pressure of 5.90x10-2 atm and a temperature of 191 K is compressed atm. Which of the following are true? constant temperature to a pressure of 116 Choose all that apply O The sample is initially a gas. O The final state of the substance is a solid. O No phase change will occur. O The sample is initially a solid. O The solid initially present will vaporize. Previnus Next
O Oth (References] Reter to the tollowing phase diagram (not to scale!) tor ammonia: 111.5 1.00 atm 0.059 195.3. 195.4 239.8 405.5 T Kolvin A sample of ammonia at a pressure of 5.90x10-2 atm and a temperature of 191 K is compressed atm. Which of the following are true? constant temperature to a pressure of 116 Choose all that apply O The sample is initially a gas. O The final state of the substance is a solid. O No phase change will occur. O The sample is initially a solid. O The solid initially present will vaporize. Previnus Next
Chemistry by OpenStax (2015-05-04)
1st Edition
ISBN:9781938168390
Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Chapter10: Liquids And Solids
Section: Chapter Questions
Problem 38E: Carbon tetrachloride, CCl4, was once used as a dry cleaning solvent, but is no longer used because...
Related questions
Question
Transcribed Image Text:= Po @
O Other Bookmarks
Reter to the tollowing phase diagram (not to scale!) tor ammonia:
[References)
111.5
1.00
atm
0.059
195.3. 195.4
239.8 405.5
T Kelvin
A sample of ammonia at a pressure of 5.90x10-2 atm and a temperature of 191 K is compressed at constant temperature to a pressure of 116
atm. Which of the following are true?
Choose all that apply
O The sample is initially a gas.
O The final state of the substance is a solid.
O No phase change will occur.
O The sample is initially a solid.
O The solid initially present will vaporize.
Previnus
Next
5:22 PM
5/5/2022
SONY
DISPLAY
WEB
![[References]
Use the References to access important values if needed for this question.
The substance krypton has the following properties:
normal melting point: 115.9 K
normal boiling point: 119.8 K
triple point:
0.72 atm, 115.8 K
critical point:
54.3 atm, 209.4 K
A sample of krypton is initially at a pressure of 58.6 atm and a temperature of 128.1 K. The pressure on the sample is reduced to 0.720 atm at a
constant temperature of 128.1 K. Which of the following are true?
Choose all that apply
O The final state of the substance is a solid.
O The liquid initially present will solidify.
O The final state of the substance is a gas.
O One or more phase changes will occur.
O The sample is initially a liquid.
Submit Answer
Try Another Version
2 item attempts remaining
Previnus
Next
5:
SONY](/v2/_next/image?url=https%3A%2F%2Fcontent.bartleby.com%2Fqna-images%2Fquestion%2F61a624e8-9805-4882-bd7c-8b439f9ba0a9%2Fabddb324-eb7c-4d9f-a532-d6f7c256d6b6%2F1rbqpbc_processed.jpeg&w=3840&q=75)
Transcribed Image Text:[References]
Use the References to access important values if needed for this question.
The substance krypton has the following properties:
normal melting point: 115.9 K
normal boiling point: 119.8 K
triple point:
0.72 atm, 115.8 K
critical point:
54.3 atm, 209.4 K
A sample of krypton is initially at a pressure of 58.6 atm and a temperature of 128.1 K. The pressure on the sample is reduced to 0.720 atm at a
constant temperature of 128.1 K. Which of the following are true?
Choose all that apply
O The final state of the substance is a solid.
O The liquid initially present will solidify.
O The final state of the substance is a gas.
O One or more phase changes will occur.
O The sample is initially a liquid.
Submit Answer
Try Another Version
2 item attempts remaining
Previnus
Next
5:
SONY
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning