Prepare a solution of NaCl by accurately weighing approximately 1.45g of NaCl into a 100 mL beaker / conical flask. Record the mass of the sample. Add 25 mL distilled water. Mix until all crystals dissolve. Prepare a solution of magnesium chloride by accurately weighing approximately 3.61 g of MgCl2٠6H20 into a 100 mL beaker / conical flask. Record the mass of the sample. Add 25 mL distilled water. Mix until all the crystals dissolve. 1. Calculate the molality of the NaCℓ and MgCℓ2.6H2O solutions. 2. Calculate the total mass of water (solvent) in kg: a) Mass of crystal water added (kg): b) Mass of water added (kg): c) Total mass of water added (kg): 3. Calculate the molality of MgCℓ2. All questions follow on from each other. Thanks very much.
Prepare a solution of NaCl by accurately weighing approximately 1.45g of NaCl into a 100 mL beaker / conical flask. Record the mass of the sample. Add 25 mL distilled water. Mix until all crystals dissolve. Prepare a solution of magnesium chloride by accurately weighing approximately 3.61 g of MgCl2٠6H20 into a 100 mL beaker / conical flask. Record the mass of the sample. Add 25 mL distilled water. Mix until all the crystals dissolve. 1. Calculate the molality of the NaCℓ and MgCℓ2.6H2O solutions. 2. Calculate the total mass of water (solvent) in kg: a) Mass of crystal water added (kg): b) Mass of water added (kg): c) Total mass of water added (kg): 3. Calculate the molality of MgCℓ2. All questions follow on from each other. Thanks very much.
Chapter2: Crystallization
Section: Chapter Questions
Problem 3Q
Related questions
Question
100%
Prepare a solution of NaCl by accurately weighing approximately 1.45g of NaCl into a 100 mL beaker / conical flask. Record the mass of the sample. Add 25 mL distilled water. Mix until all crystals dissolve.
Prepare a solution of magnesium chloride by accurately weighing approximately 3.61 g of MgCl2٠6H20 into a 100 mL beaker / conical flask. Record the mass of the sample. Add 25 mL
distilled water. Mix until all the crystals dissolve.
1. Calculate the molality of the NaCℓ and MgCℓ2.6H2O solutions.
2. Calculate the total mass of water (solvent) in kg:
- a) Mass of crystal water added (kg):
- b) Mass of water added (kg):
- c) Total mass of water added (kg):
3. Calculate the molality of MgCℓ2.
All questions follow on from each other. Thanks very much.
Transcribed Image Text:12
10
4
2
-2
H2O
-4
Time (s)
Na CI
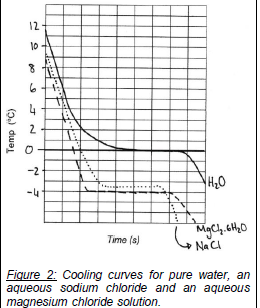
Figure 2: Cooling curves for pure water, an
aqueous sodium chloride and an aqueous
magnesium chloride solution.
(5.) dwal
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

EBK A SMALL SCALE APPROACH TO ORGANIC L
Chemistry
ISBN:
9781305446021
Author:
Lampman
Publisher:
CENGAGE LEARNING - CONSIGNMENT


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

EBK A SMALL SCALE APPROACH TO ORGANIC L
Chemistry
ISBN:
9781305446021
Author:
Lampman
Publisher:
CENGAGE LEARNING - CONSIGNMENT


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

