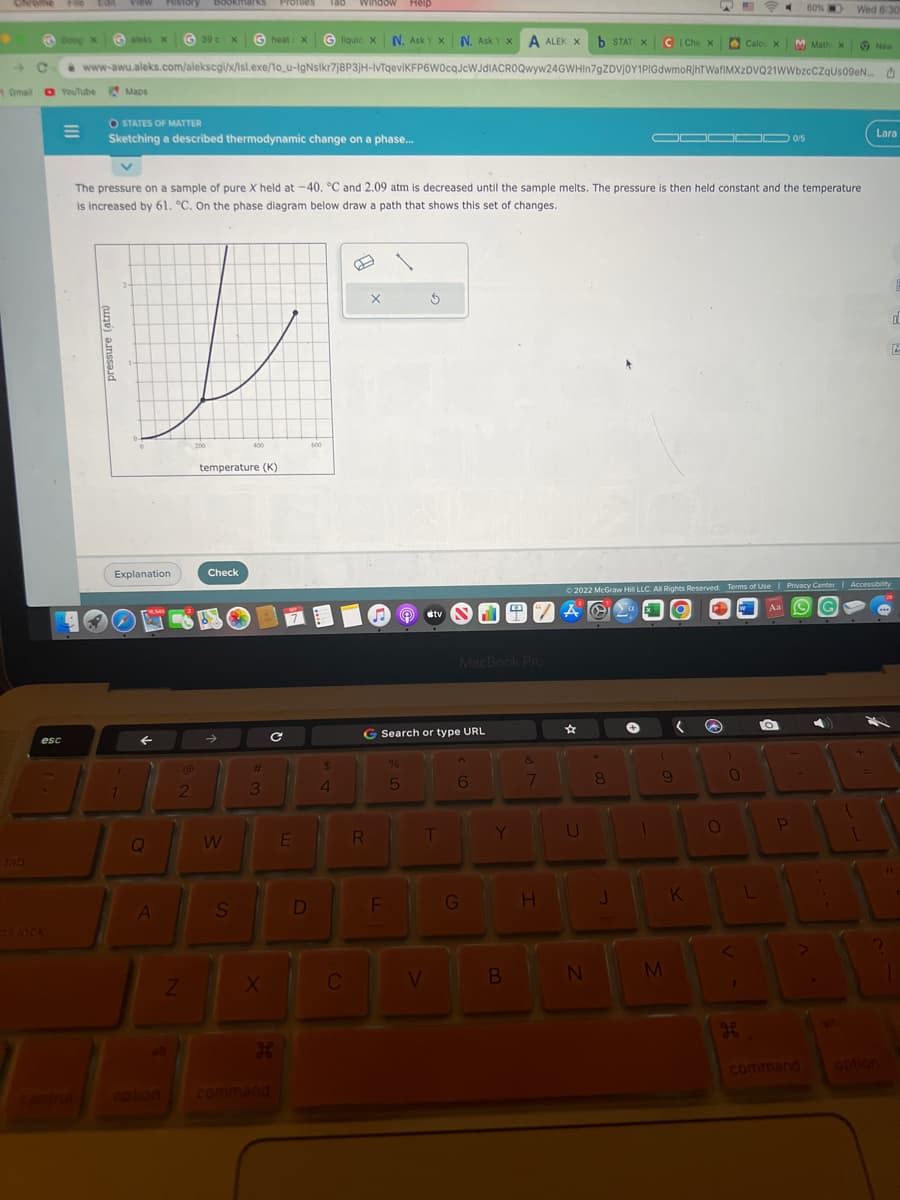
O STATES OF MATTER Sketching a described thermodynamic change on a phase... The pressure on a sample of pure X held at -40. °C and 2.09 atm is decreased until the sample melts. The pressure is then held constant and the temperature is increased by 61. °C. On the phase diagram below draw a path that shows this set of changes. pressure (atm) 200 400 temperature (K) 0/5 600 La
O STATES OF MATTER Sketching a described thermodynamic change on a phase... The pressure on a sample of pure X held at -40. °C and 2.09 atm is decreased until the sample melts. The pressure is then held constant and the temperature is increased by 61. °C. On the phase diagram below draw a path that shows this set of changes. pressure (atm) 200 400 temperature (K) 0/5 600 La
Chemistry: Principles and Reactions
8th Edition
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:William L. Masterton, Cecile N. Hurley
Chapter1: Matter And Measurements
Section: Chapter Questions
Problem 56QAP: Magnesium chloride is an important coagulant used in the preparation of tofu from soy milk. Its...
Related questions
Question
Transcribed Image Text:tab
File
esc
ns lock
Ooop X
39 c) x Gheat
heat X Ⓒliquid X N. AskYX N. AskYX A ALEK X b STAT X CI Che X
C www-awu.aleks.com/alekscgi/x/lsl.exe/1o_u-IgNslkr7j8P3jH-IvTqeviKFP6W0cqJcWJdIACROQwyw24GWHIn7gZDVjOY1PIGdwmoRjhTWafIMXzDVQ21WWbzcCZqUs09eN...
Omall O YouTube Maps
View History bookmarks Profiles 180 window Help
O STATES OF MATTER
Sketching a described thermodynamic change on a phase...
pressure (atm)
aleks X
1
Explanation
←
v
The pressure on a sample of pure X held at -40. °C and 2.09 atm is decreased until the sample melts. The pressure is then held constant and the temperature
is increased by 61. °C. On the phase diagram below draw a path that shows this set of changes.
Q
A
2
Z
200
temperature (K)
Check
W
400
S
#
3
X
C с
H
E
D
600
$
4
C
X
R
F
%
5
S
G Search or type URL
stv
V
T
F
MacBook Pro
6
G
4
Y
B
&
7
H
☆
U
N
*
8
J
+
O▬▬▬▬0/5
Ⓒ2022 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center | Accessibility
XO
AO
W
(
9
M
( @
K
O
0
Calcu X
<
1
He
60%
L
M Math X
Aa
Wed 6:30
P
New
1
D
Lara
0
11
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning


Chemistry for Today: General, Organic, and Bioche…
Chemistry
ISBN:
9781305960060
Author:
Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning


Chemistry for Today: General, Organic, and Bioche…
Chemistry
ISBN:
9781305960060
Author:
Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning