O STATES OF MATTER Understanding how solubility varies with temperature and. Predict V will be observed in each experiment below. predicted observation (choose one) experiment O A bigger mass of NaCl precipitate will form in Sample #2. Two 250 mL samples of water are drawn from a deep well bored into a large underground salt (NaCI) deposit. Sample #1 is from the top of the well, and is initially at 42 °C. Sample #2 is from a depth of 150 m, and is initially at 8C. Both samples are allowed to come to room temperature (20 "C) and 1 atm pressure. An NaCI precipitate is seen to form in Sample #1. OA smaller mass of NaCl precipitate will form in Sample #2. O The same mass of NaCl precipitate will form in Sample #2. No precipitate will form in Sample #2. I need more information to predict whether and how much precipitate wii form in Sample #2. Can A will make a louder and stronger fizz than can B. A student has two unopened 33 cL cans containing carbonated water. Can A has been stored in the garage (32 °C) and can B has been stored in the fridge (8 °C). The student opens one can at the time, both cans make a fizz. O Can B will make a louder and stronger fizz than can A. O The fizz will be the same for both cans. There is not enough information to predict which can will make the louder fizz.
O STATES OF MATTER Understanding how solubility varies with temperature and. Predict V will be observed in each experiment below. predicted observation (choose one) experiment O A bigger mass of NaCl precipitate will form in Sample #2. Two 250 mL samples of water are drawn from a deep well bored into a large underground salt (NaCI) deposit. Sample #1 is from the top of the well, and is initially at 42 °C. Sample #2 is from a depth of 150 m, and is initially at 8C. Both samples are allowed to come to room temperature (20 "C) and 1 atm pressure. An NaCI precipitate is seen to form in Sample #1. OA smaller mass of NaCl precipitate will form in Sample #2. O The same mass of NaCl precipitate will form in Sample #2. No precipitate will form in Sample #2. I need more information to predict whether and how much precipitate wii form in Sample #2. Can A will make a louder and stronger fizz than can B. A student has two unopened 33 cL cans containing carbonated water. Can A has been stored in the garage (32 °C) and can B has been stored in the fridge (8 °C). The student opens one can at the time, both cans make a fizz. O Can B will make a louder and stronger fizz than can A. O The fizz will be the same for both cans. There is not enough information to predict which can will make the louder fizz.
Chapter79: Solubility
Section: Chapter Questions
Problem 1P
Related questions
Question
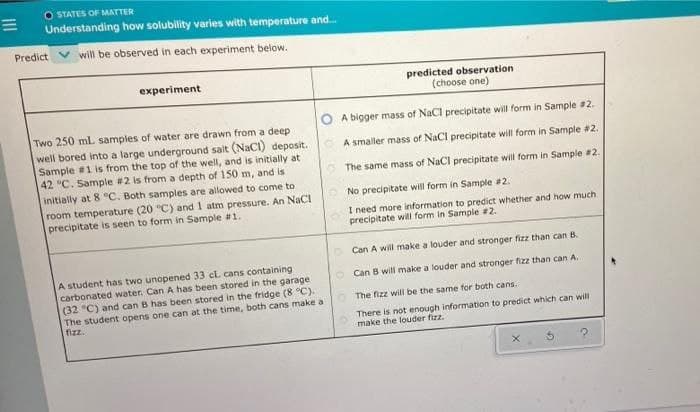
Transcribed Image Text:O STATES OF MATTER
Understanding how solubility varies with temperature and.
Predict V will be observed in each experiment below.
experiment
predicted observation
(choose one)
O A bigger mass of NaCl precipitate will form in Sample 2.
Two 250 mL samples of water are drawn from a deep
well bored into a large underground salt (NaCI) deposit.
Sample #1 is from the top of the well, and is initially at
42 °C. Sample #2 is from a depth of 150 m, and is
initially at 8 C. Both samples are allowed to come to
room temperature (20 "C) and 1 atm pressure. An NaCI
precipitate is seen to form in Sample #1.
A smaller mass of NaCl precipitate will form in Sample #2.
The same mass of NaCl precipitate will form in Sample #2.
No precipitate will form in Sample 2.
I need more information to predict whether and how much
precipitate will form in Sample #2.
Can A will make a louder and stronger fizz than can B.
A student has two unopened 33 cL cans containing
carbonated water. Can A has been stored in the garage
(32 °C) and can B has been stored in the fridge (8 °C).
The student opens one can at the time, both cans make a
fizz.
Can B will make a louder and stronger fizz than can A.
O The fizz will be the same for both cans.
There is not enough information to predict which can will
make the louder fizz.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

EBK A SMALL SCALE APPROACH TO ORGANIC L
Chemistry
ISBN:
9781305446021
Author:
Lampman
Publisher:
CENGAGE LEARNING - CONSIGNMENT

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


EBK A SMALL SCALE APPROACH TO ORGANIC L
Chemistry
ISBN:
9781305446021
Author:
Lampman
Publisher:
CENGAGE LEARNING - CONSIGNMENT

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning