Part A Rank these systems in order of decreasing entropy. Rank from highest to lowest entropy. To rank items as equivalent, overlap them. > Vlew Available Hint(s) Reset Help 1 mol of carbon tetrachloride gas at 1/2 mol of neon gas at 100 K and 20 L 1 mol of nitrogen gas at 273 K and 40L 1 mol of neon gas at 273 Kand 20L 1 mol of neon gas at 273 Kand 40 L 1/2 mol of neon gas at 273 Kand 20 L 12 mot of liquid neon at 100 K 273 K and 4O L Greatest entropy Least entropy The correct ranking cannot be determined.
Part A Rank these systems in order of decreasing entropy. Rank from highest to lowest entropy. To rank items as equivalent, overlap them. > Vlew Available Hint(s) Reset Help 1 mol of carbon tetrachloride gas at 1/2 mol of neon gas at 100 K and 20 L 1 mol of nitrogen gas at 273 K and 40L 1 mol of neon gas at 273 Kand 20L 1 mol of neon gas at 273 Kand 40 L 1/2 mol of neon gas at 273 Kand 20 L 12 mot of liquid neon at 100 K 273 K and 4O L Greatest entropy Least entropy The correct ranking cannot be determined.
Chemistry: An Atoms First Approach
2nd Edition
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Steven S. Zumdahl, Susan A. Zumdahl
Chapter16: Spontaneity, Entropy, And Free Energy
Section: Chapter Questions
Problem 5ALQ
Related questions
Question
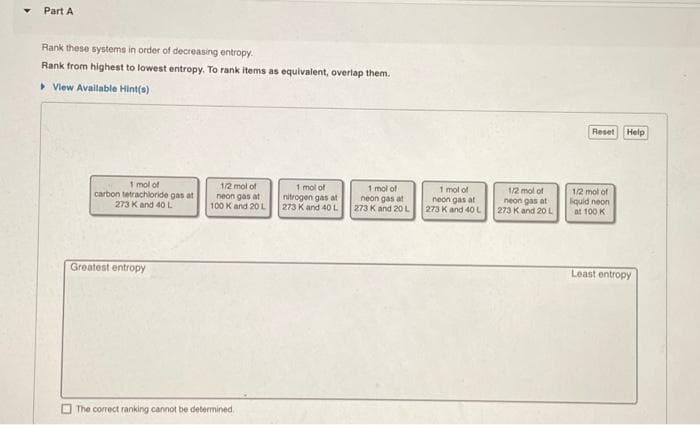
Transcribed Image Text:Part A
Rank these systems in order of decreasing entropy.
Rank from highest to lowest entropy. To rank items as equivalent, overlap them.
> Vlew Available Hint(s)
Reset
Help
1 mol of
carbon tetrachloride gas at
273 Kand 40 L
1/2 mol of
neon gas at
100 K and 20 L
1 mol of
nitrogen gas at
273 K and 40L
1 mol of
neon gas at
273 K and 20L
1 mol of
neon gas at
273 K and 4OL
1/2 mol of
neon gas at
273 K and 20 L
1/2 mol of
liquid neon
at 100 K
Greatest entropy
Least entropy
O The correct ranking cannot be determined,
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning