O THERMOCHEMISTRY Understanding the definition of enthalpy Measurements show that the enthalpy of a mixture of gaseous reactants decreases by 278. kJ during a certain chemical reaction, which is carried out at a constant pressure. Furthermore, by carefully monitoring the volume change it is determined that -83. kJ of work is done on the mixture during the reaction. Calculate the change in energy of the gas mixture during the reaction. Be sure your answer has the correct number of significant digits. O exothermic Is the reaction exothermic or endothermic? O endothermic
O THERMOCHEMISTRY Understanding the definition of enthalpy Measurements show that the enthalpy of a mixture of gaseous reactants decreases by 278. kJ during a certain chemical reaction, which is carried out at a constant pressure. Furthermore, by carefully monitoring the volume change it is determined that -83. kJ of work is done on the mixture during the reaction. Calculate the change in energy of the gas mixture during the reaction. Be sure your answer has the correct number of significant digits. O exothermic Is the reaction exothermic or endothermic? O endothermic
Chemistry for Engineering Students
4th Edition
ISBN:9781337398909
Author:Lawrence S. Brown, Tom Holme
Publisher:Lawrence S. Brown, Tom Holme
Chapter9: Energy And Chemistry
Section: Chapter Questions
Problem 9.101PAE
Related questions
Question
be sure your answer is correct and have the correct number of significant digits
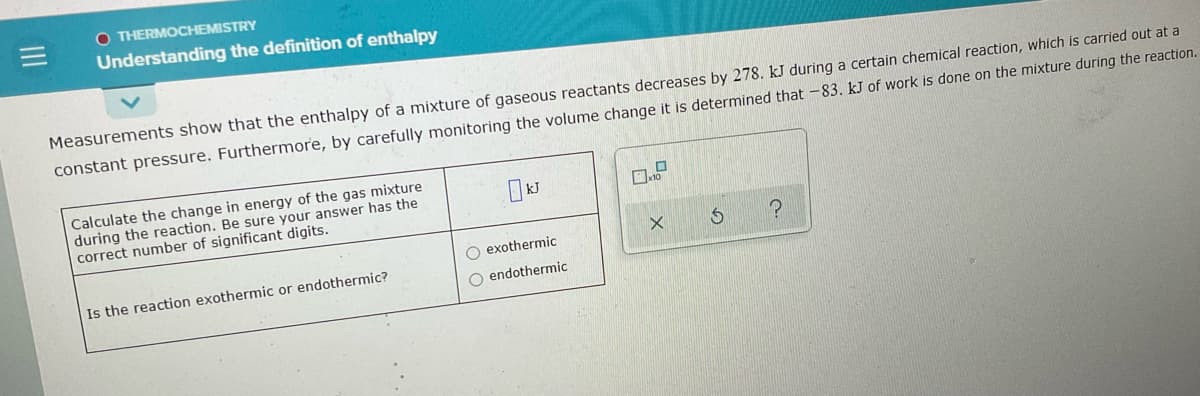
Transcribed Image Text:O THERMOCHEMISTRY
Understanding the definition of enthalpy
Measurements show that the enthalpy of a mixture of gaseous reactants decreases by 278. kJ during a certain chemical reaction, which is carried out at a
constant pressure. Furthermore, by carefully monitoring the volume change it is determined that -83. kJ of work is done on the mixture during the reaction.
Calculate the change in energy of the gas mixture
during the reaction. Be sure your answer has the
correct number of significant digits.
O exothermic
Is the reaction exothermic or endothermic?
O endothermic
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax