O Using the equilibrium shown below, fill in the table showing which way each stress would shift things to re-establish equilibrium and the effect on the value of Kc each stress will have. Assume that only the stress that is being applied is happening to the system at equilibrium and nothing else changes (e.g. the volume of the container remains constant unless you are told otherwise). AH = -60 kJ 2CHCI3(g) +Cl2(g) = 2CC14(1) + H2(g) Change in the value of Кс Direction of shift to re- Stress establish equilibrium Add Cl2e) Add CCl40) Remove CHCI3(g) Cool System Add a catalyst Reduce volume Add Heg)
O Using the equilibrium shown below, fill in the table showing which way each stress would shift things to re-establish equilibrium and the effect on the value of Kc each stress will have. Assume that only the stress that is being applied is happening to the system at equilibrium and nothing else changes (e.g. the volume of the container remains constant unless you are told otherwise). AH = -60 kJ 2CHCI3(g) +Cl2(g) = 2CC14(1) + H2(g) Change in the value of Кс Direction of shift to re- Stress establish equilibrium Add Cl2e) Add CCl40) Remove CHCI3(g) Cool System Add a catalyst Reduce volume Add Heg)
Chemistry for Engineering Students
4th Edition
ISBN:9781337398909
Author:Lawrence S. Brown, Tom Holme
Publisher:Lawrence S. Brown, Tom Holme
Chapter12: Chemical Equilibrium
Section: Chapter Questions
Problem 12.83PAE
Related questions
Question
Can someone explain to me how do I do this question? Please
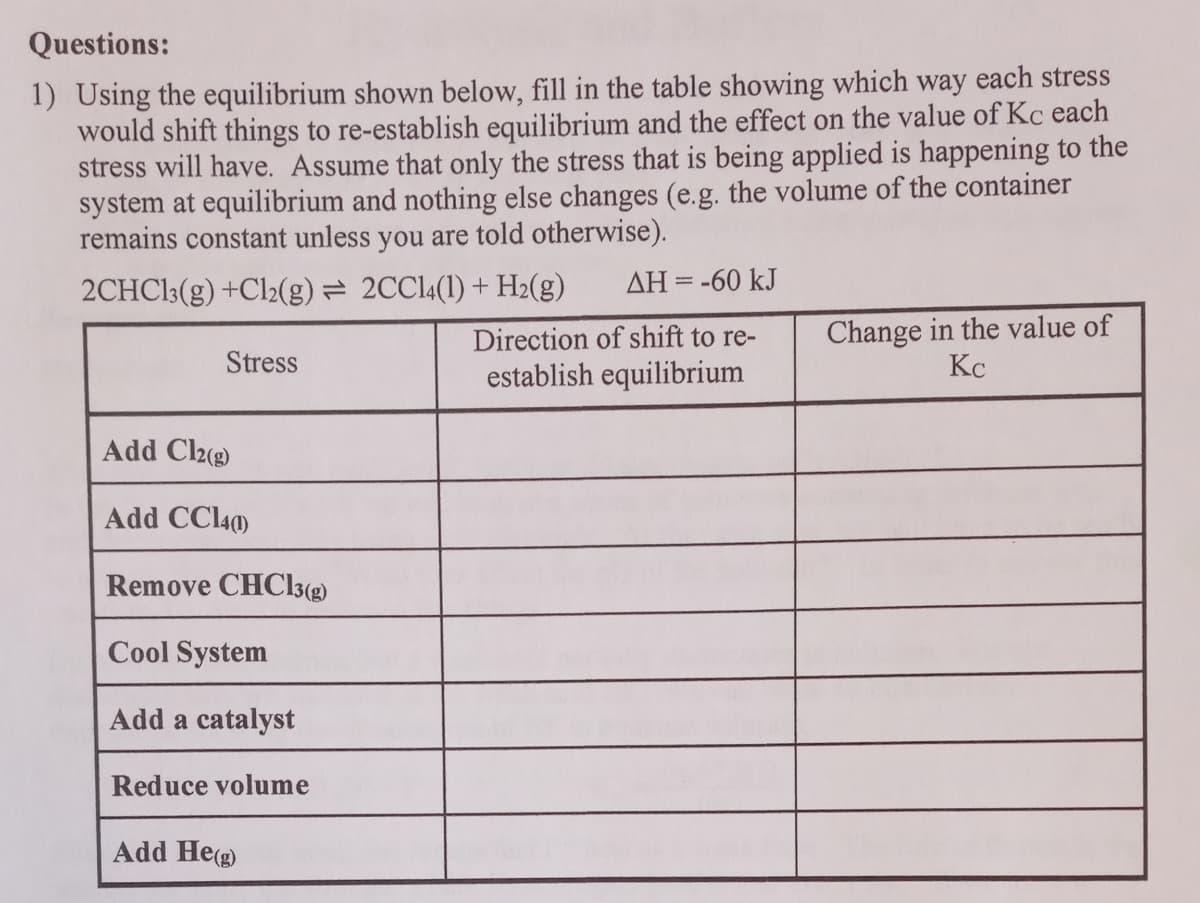
Transcribed Image Text:Questions:
1) Using the equilibrium shown below, fill in the table showing which way each stress
would shift things to re-establish equilibrium and the effect on the value of Kc each
stress will have. Assume that only the stress that is being applied is happening to the
system at equilibrium and nothing else changes (e.g. the volume of the container
remains constant unless you are told otherwise).
2CHC13(g) +Cl2(g) = 2CC14(1) + H2(g)
ΔΗ--60 kJ
Change in the value of
Kc
Direction of shift to re-
Stress
establish equilibrium
Add Cl2(g)
Add CCl40)
Remove CHC3(g)
Cool System
Add a catalyst
Reduce volume
Add Heg)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning