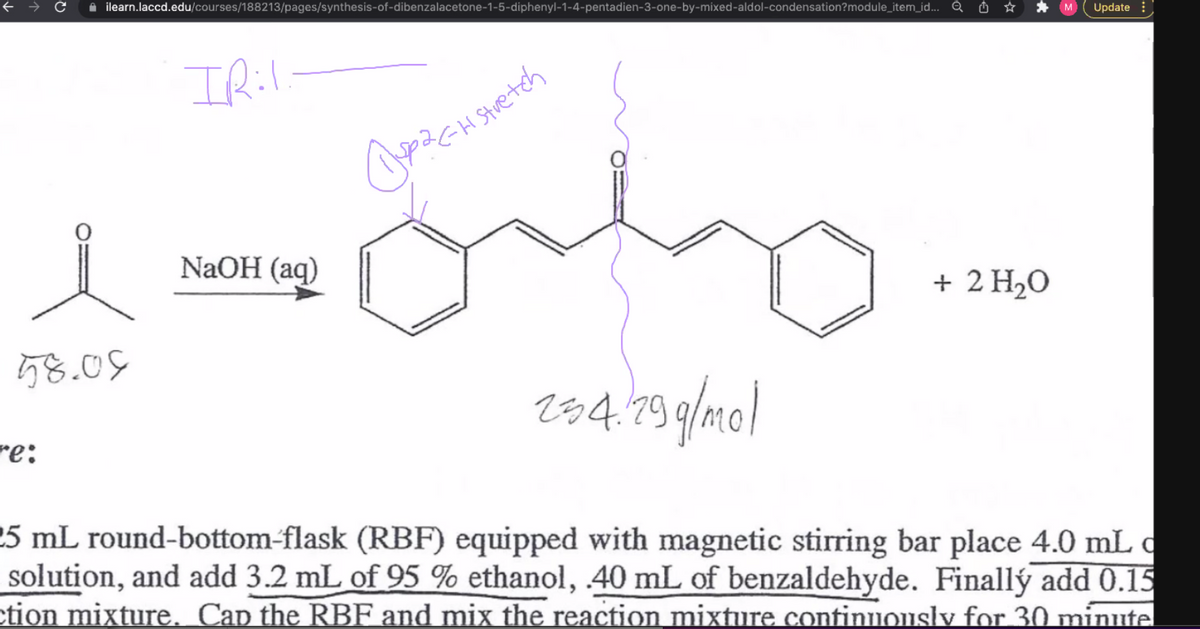
IRil. 4 Stretch NaOH (aq) + 2 H2O 58.05 t84.79 g/me| :a 5 mL round-bottom-flask (RBF) equipped with magnetic stirring bar place 4.0 mL c solution, and add 3.2 mL of 95 % ethanol, 40 mL of benzaldehyde. Finallý add 0.15
IRil. 4 Stretch NaOH (aq) + 2 H2O 58.05 t84.79 g/me| :a 5 mL round-bottom-flask (RBF) equipped with magnetic stirring bar place 4.0 mL c solution, and add 3.2 mL of 95 % ethanol, 40 mL of benzaldehyde. Finallý add 0.15
Organic Chemistry
8th Edition
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Chapter19: Enolate Anions And Enamines
Section: Chapter Questions
Problem 19.55P
Related questions
Question
I need help predcting the 1hnmr of the product and give the chemical shift and mutiplcity of for each proton
Transcribed Image Text:1 ilearn.laccd.edu/courses/188213/pages/synthesis-of-dibenzalacetone-1-5-diphenyl-1-4-pentadien-3-one-by-mixed-aldol-condensation?module_item_id... Q O *
* M
Update
Stuetch
NaOH (aq)
+ 2 H20
58.09
284.19 9/mol
re:
25 mL round-bottom-flask (RBF) equipped with magnetic stirring bar place 4.0 mL c
solution, and add 3.2 mL of 95 % ethanol, 40 mL of benzaldehyde. Finallý add 0.15
ction mixture. Cap the RBF and mix the reaction mixture continuonsly for 30 minute
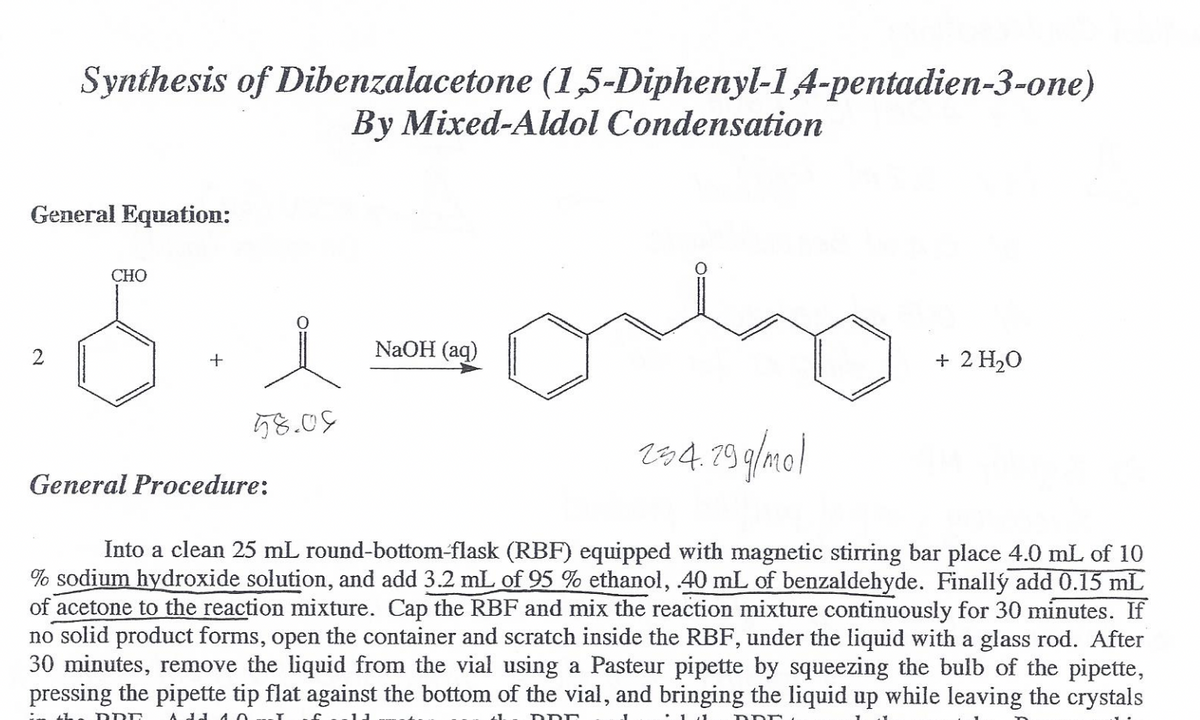
Transcribed Image Text:Synthesis of Dibenzalacetone (1,5-Diphenyl-1A-pentadien-3-one)
By Mixed-Aldol Condensation
General Equation:
CHO
NaOH (aq)
+ 2 H20
58.05
r84. 79 g/mo)
General Procedure:
Into a clean 25 mL round-bottom-flask (RBF) equipped with magnetic stirring bar place 4.0 mL of 10
% sodium hydroxide solution, and add 3.2 mL of 95 % ethanol, 40 mL of benzaldehyde. Finallý add 0.15 mL
of acetone to the reaction mixture. Cap the RBF and mix the reaction mixture continuously for 30 minutes. If
no solid product forms, open the container and scratch inside the RBF, under the liquid with a glass rod. After
30 minutes, remove the liquid from the vial using a Pasteur pipette by squeezing the bulb of the pipette,
pressing the pipette tip flat against the bottom of the vial, and bringing the liquid up while leaving the crystals
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps with 4 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning


Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning
