Observations of Demonstrations 1. Combustion of alcohols. Observe the color and characteristics of the flames: c. 2-propanol (CH,CHCH,) 1. OH Methanol (CH,OH) b. Ethanol (CH,CH,OH) a. What trend is observed as the number of carbons in the chain increases? Do think that it is possible that products other than carbon dioxide and water vapor are pro- you duced when these substances burn? What would make you suspect this? CH,CH OH(1) + 3 0,(g) – 2 CO,(g) + 3 H,O(g) (Combustion of ethanol)
Observations of Demonstrations 1. Combustion of alcohols. Observe the color and characteristics of the flames: c. 2-propanol (CH,CHCH,) 1. OH Methanol (CH,OH) b. Ethanol (CH,CH,OH) a. What trend is observed as the number of carbons in the chain increases? Do think that it is possible that products other than carbon dioxide and water vapor are pro- you duced when these substances burn? What would make you suspect this? CH,CH OH(1) + 3 0,(g) – 2 CO,(g) + 3 H,O(g) (Combustion of ethanol)
Chemistry: The Molecular Science
5th Edition
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:John W. Moore, Conrad L. Stanitski
Chapter3: Chemical Reactions
Section3.5: Oxidatino-reduction And Electron Transfer
Problem 3.14CE
Related questions
Question
Observations of demonstrations, help answer the combustion’s of alcohols, and answer the following two questions relating to observations of demonstrations.
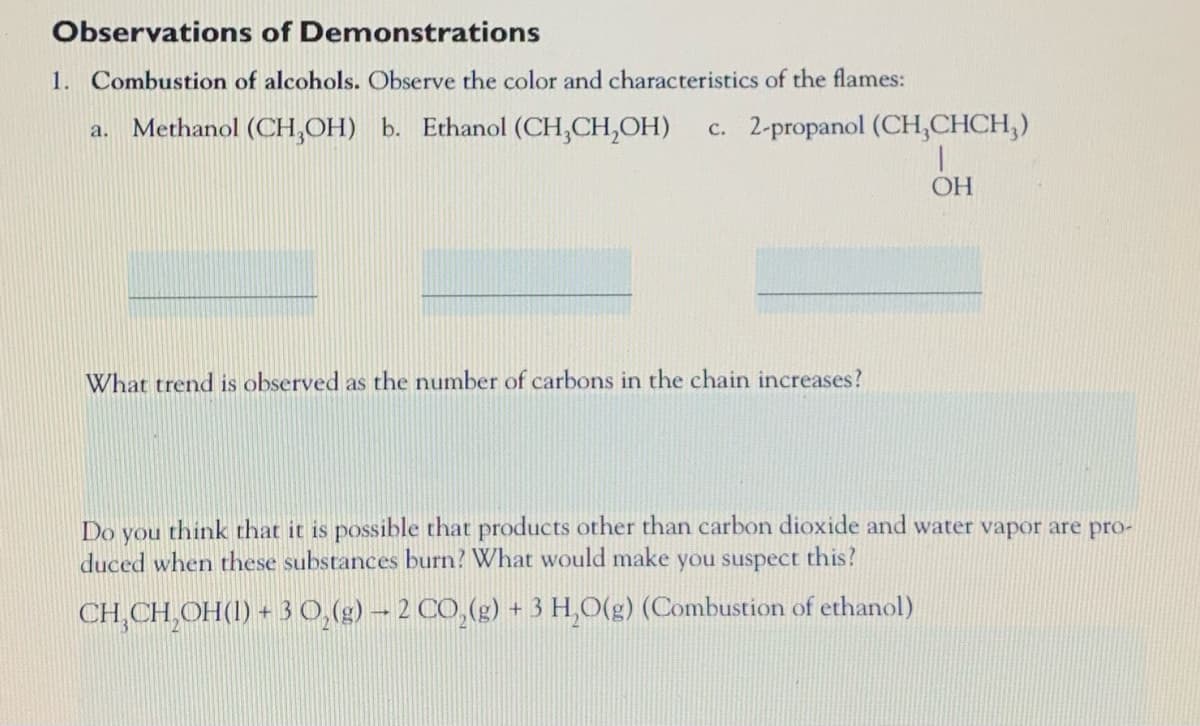
Transcribed Image Text:Observations of Demonstrations
1. Combustion of alcohols. Observe the color and characteristics of the flames:
Methanol (CH,OH) b. Ethanol (CH,CH,OH)
c. 2-propanol (CH,CHCH,)
a.
OH
What trend is observed as the number of carbons in the chain increases?
Do
think that it is possible that products other than carbon dioxide and water vapor are pro-
you
duced when these substances burn? What would make
you suspect this?
CH,CH,OH(1) + 3 0,(g) – 2 CO,(g) + 3 H,O(g) (Combustion of ethanol)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Living By Chemistry: First Edition Textbook
Chemistry
ISBN:
9781559539418
Author:
Angelica Stacy
Publisher:
MAC HIGHER

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Living By Chemistry: First Edition Textbook
Chemistry
ISBN:
9781559539418
Author:
Angelica Stacy
Publisher:
MAC HIGHER

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning