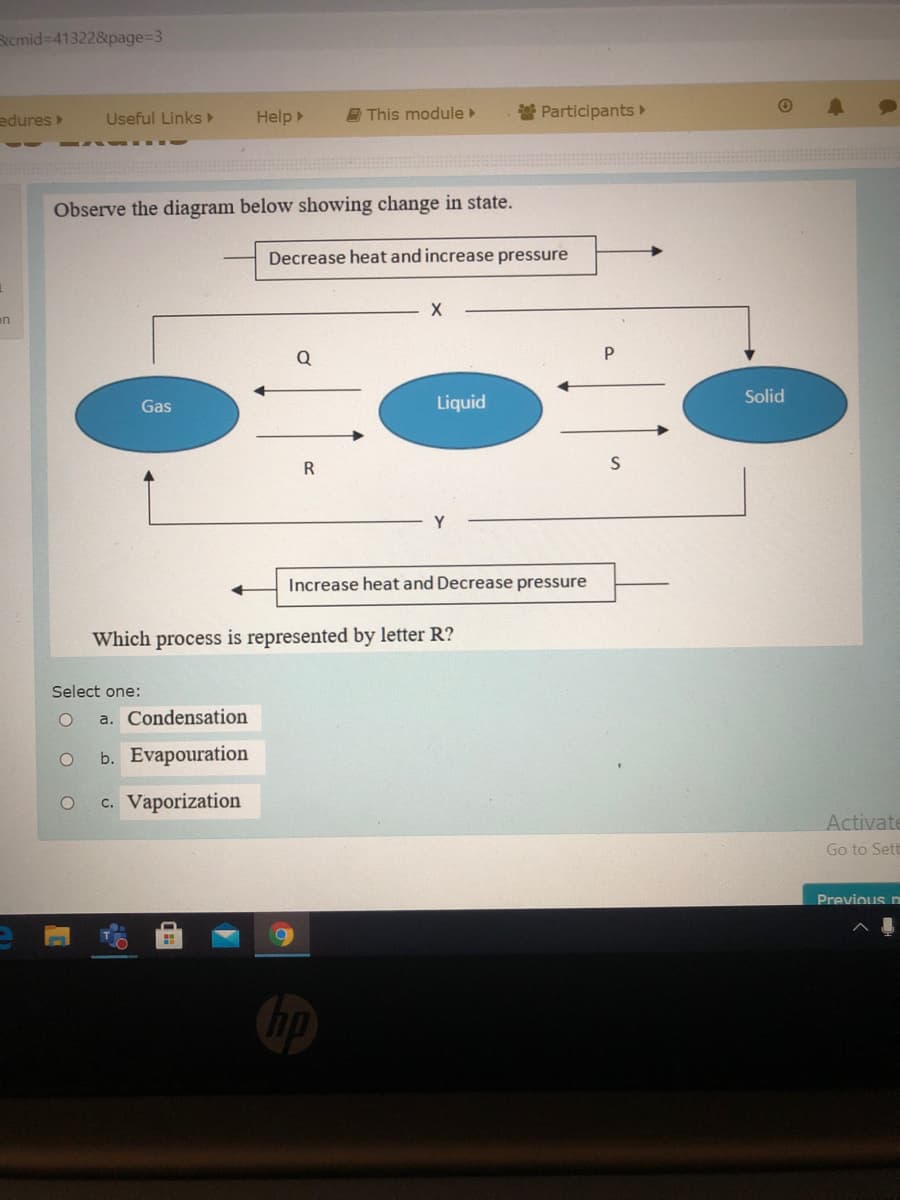
Observe the diagram belów showing ehange Decrease heat and increase pressure - Q P Solid Gas Liquid R Y Increase heat and Decrease pressure Which process is represented by letter R? Select one: a. Condensation b. Evapouration O c. Vaporization Act 11
Observe the diagram belów showing ehange Decrease heat and increase pressure - Q P Solid Gas Liquid R Y Increase heat and Decrease pressure Which process is represented by letter R? Select one: a. Condensation b. Evapouration O c. Vaporization Act 11
Chapter1: Temperature And Heat
Section: Chapter Questions
Problem 16CQ: As shown below, which is the phase diagram for carbon dioxide, what is the vapor pressure of solid...
Related questions
Question
Transcribed Image Text:Rcmid=41322&page%=D3
edures
Useful Links
Help
E This module
Participants►
Observe the diagram below showing change in state.
Decrease heat and increase pressure
en
Q
Solid
Gas
Liquid
R
Increase heat and Decrease pressure
Which process is represented by letter R?
Select one:
a. Condensation
b. Evapouration
c. Vaporization
Activate
Go to Set
Previous n
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, physics and related others by exploring similar questions and additional content below.Recommended textbooks for you


College Physics
Physics
ISBN:
9781938168000
Author:
Paul Peter Urone, Roger Hinrichs
Publisher:
OpenStax College



College Physics
Physics
ISBN:
9781938168000
Author:
Paul Peter Urone, Roger Hinrichs
Publisher:
OpenStax College
