Ocr „H,OH being created or destroyed by the chemical ction? Ode O ne „H,OH is being created or destroyed, what is the e at which it is being created or destroyed 100 seconds er the reaction starts? ind your answer to 2 significant digits. Also be sure r answer has the correct unit symbol. „H,OH is being created or destroyed, what is the erage rate at which it is being created or destroyed ing the first 100 seconds of the reaction? ind your answer to 2 significant digits. Also be sure r answer has the correct unit symbol.
Ocr „H,OH being created or destroyed by the chemical ction? Ode O ne „H,OH is being created or destroyed, what is the e at which it is being created or destroyed 100 seconds er the reaction starts? ind your answer to 2 significant digits. Also be sure r answer has the correct unit symbol. „H,OH is being created or destroyed, what is the erage rate at which it is being created or destroyed ing the first 100 seconds of the reaction? ind your answer to 2 significant digits. Also be sure r answer has the correct unit symbol.
Chemistry: Matter and Change
1st Edition
ISBN:9780078746376
Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Chapter9: Chemical Reactions
Section: Chapter Questions
Problem 139A
Related questions
Question
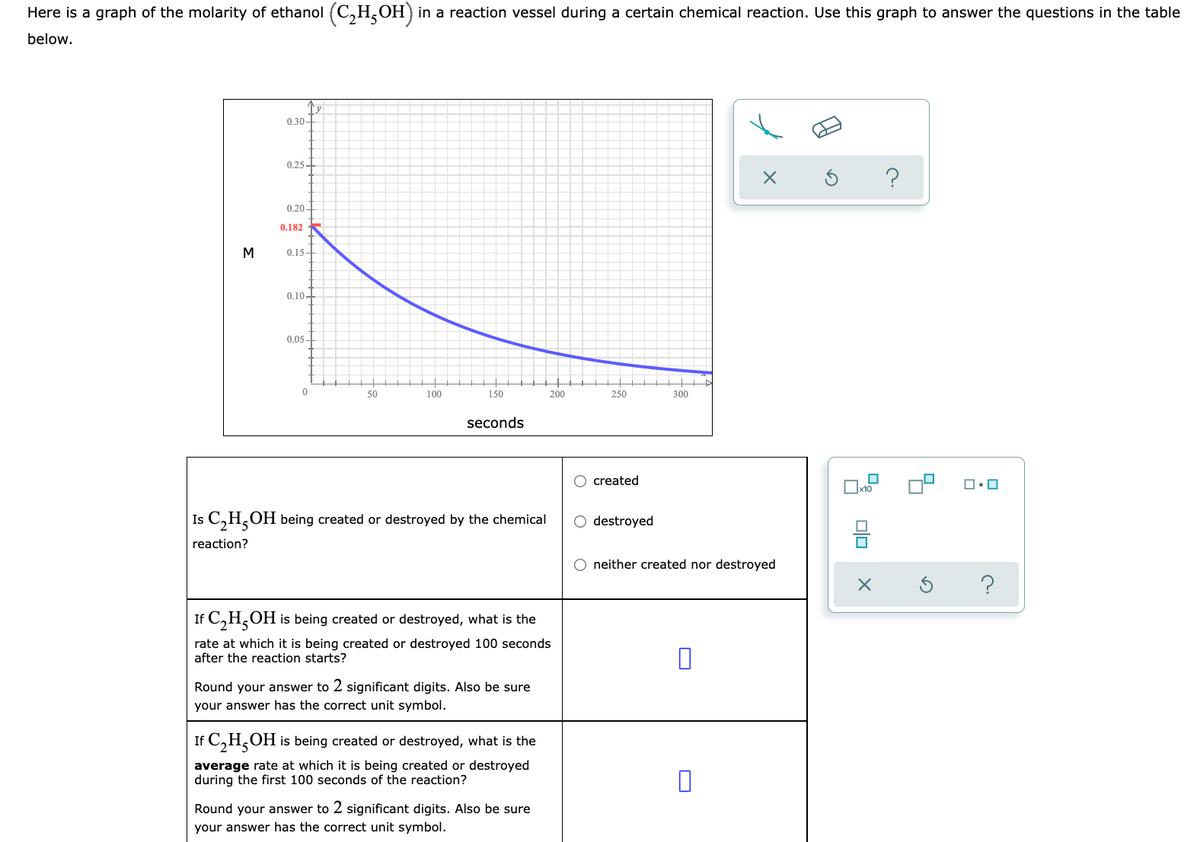
Transcribed Image Text:Here is a graph of the molarity of ethanol (C,H,OH) in a reaction vessel during a certain chemical reaction. Use this graph to answer the questions in the table
below.
0.30-
0.25+
0.20-
0.182
M
0.15-
0.10-
0.05 -
50
100
150
200
250
300
seconds
O created
x10
Is C,H,OH being created or destroyed by the chemical
O destroyed
reaction?
neither created nor destroyed
If C,H,OH is being created or destroyed, what is the
rate at which it is being created or destroyed 100 seconds
after the reaction starts?
Round your answer to 2 significant digits. Also be sure
your answer has the correct unit symbol.
If C,H,OH is being created or destroyed, what is the
average rate at which it is being created or destroyed
during the first 100 seconds of the reaction?
Round your answer to 2 significant digits. Also be sure
your answer has the correct unit symbol.
Expert Solution
Step 1
We are given a graph showing change in concentration with ethanol w.r.t time.
We have to tell whether ethanol is being created or destroyed and hence its rate.
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

World of Chemistry
Chemistry
ISBN:
9780618562763
Author:
Steven S. Zumdahl
Publisher:
Houghton Mifflin College Div

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

World of Chemistry
Chemistry
ISBN:
9780618562763
Author:
Steven S. Zumdahl
Publisher:
Houghton Mifflin College Div

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning