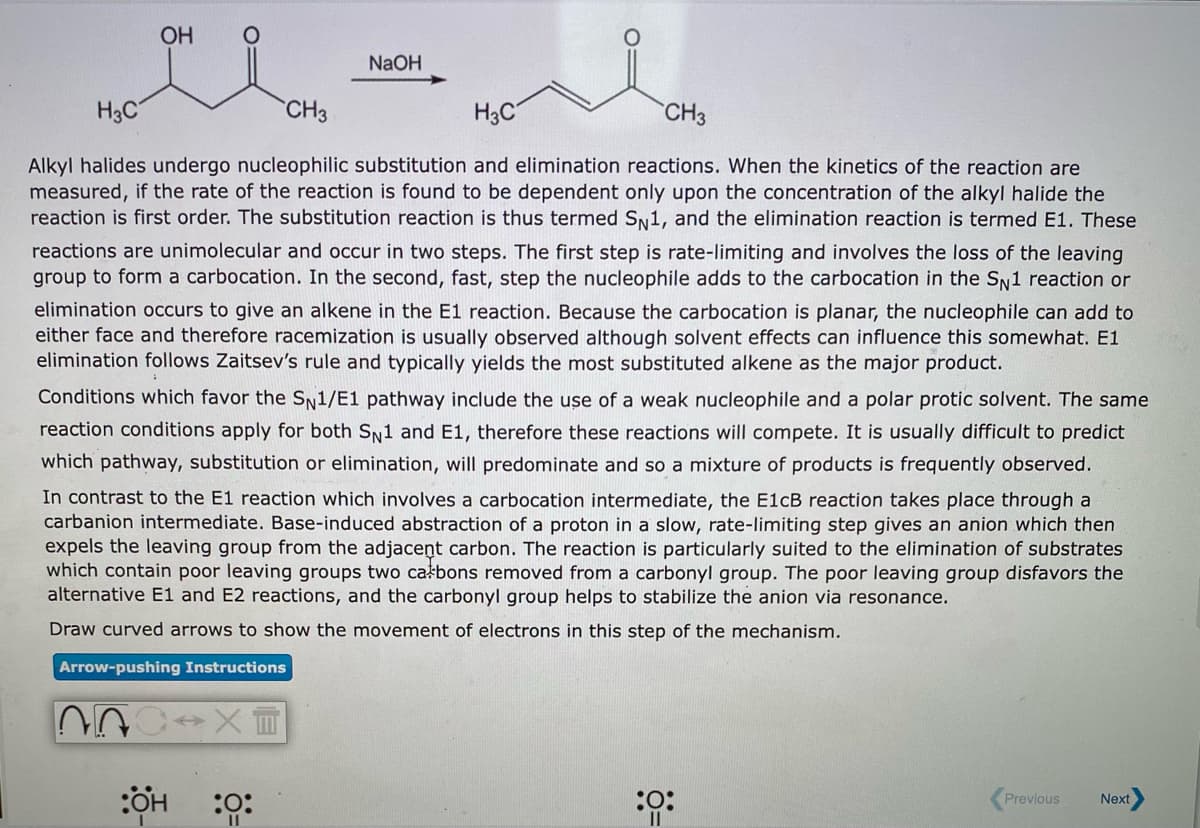
OH NaOH H3C CH3 H3C CH3 Alkyl halides undergo nucleophilic substitution and elimination reactions. When the kinetics of the reaction are measured, if the rate of the reaction is found to be dependent only upon the concentration of the alkyl halide the reaction is first order. The substitution reaction is thus termed SN1, and the elimination reaction is termed E1. These reactions are unimolecular and occur in two steps. The first step is rate-limiting and involves the loss of the leaving group to form a carbocation. In the second, fast, step the nucleophile adds to the carbocation in the SN1 reaction or elimination occurs to give an alkene in the E1 reaction. Because the carbocation is planar, the nucleophile can add to either face and therefore racemization is usually observed although solvent effects can influence this somewhat. E1 elimination follows Zaitsev's rule and typically yields the most substituted alkene as the major product. Conditions which favor the SN1/E1 pathway include the use of a weak nucleophile and a polar protic solvent. The same reaction conditions apply for both SN1 and E1, therefore these reactions will compete. It is usually difficult to predict which pathway, substitution or elimination, will predominate and so a mixture of products is frequently observed. In contrast to the E1 reaction which involves a carbocation intermediate, the E1CB reaction takes place through a carbanion intermediate. Base-induced abstraction of a proton in a slow, rate-limiting step gives an anion which then expels the leaving group from the adjacent carbon. The reaction is particularly suited to the elimination of substrates which contain poor leaving groups two całbons removed from a carbonyl group. The poor leaving group disfavors the alternative E1 and E2 reactions, and the carbonyl group helps to stabilize the anion via resonance. Draw curved arrows to show the movement of electrons in this step of the mechanism.
Reactions of Ethers
Ethers (R-O-R’) are compounds formed by replacing hydrogen atoms of an alcohol (R-OH compound) or a phenol (C6H5OH) by an aryl/ acyl group (functional group after removing single hydrogen from an aromatic ring). In this section, reaction, preparation and behavior of ethers are discussed in the context of organic chemistry.
Epoxides
Epoxides are a special class of cyclic ethers which are an important functional group in organic chemistry and generate reactive centers due to their unusual high reactivity. Due to their high reactivity, epoxides are considered to be toxic and mutagenic.
Williamson Ether Synthesis
An organic reaction in which an organohalide and a deprotonated alcohol forms ether is known as Williamson ether synthesis. Alexander Williamson developed the Williamson ether synthesis in 1850. The formation of ether in this synthesis is an SN2 reaction.
Step by step
Solved in 2 steps with 5 images


