ON 22 given pure samples of ammonia, NH3(g), and nitrogen trifluoride, NF3(g) What prediction would you make concerning their st ven if more conditions are specified, a reliable prediction cannot be made ammonia
ON 22 given pure samples of ammonia, NH3(g), and nitrogen trifluoride, NF3(g) What prediction would you make concerning their st ven if more conditions are specified, a reliable prediction cannot be made ammonia
Chapter31: Introduction To Analytical Separations
Section: Chapter Questions
Problem 31.33QAP
Related questions
Concept explainers
Question
100%
Pls help ASAP
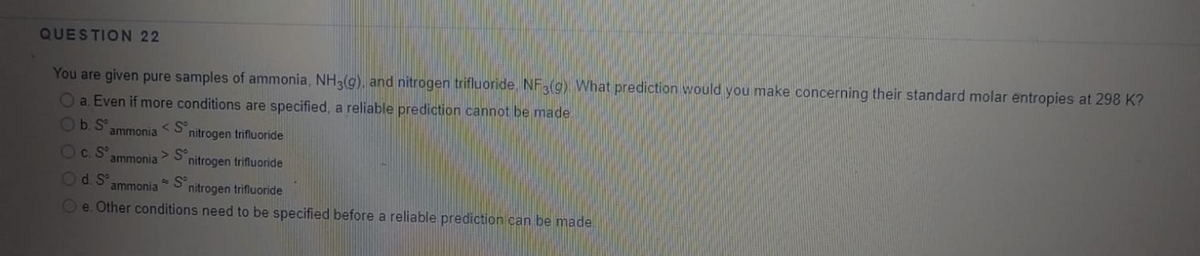
Transcribed Image Text:QUESTION 22
You are given pure samples of ammonia, NH3(g), and nitrogen trifluoride. NF3(g) What prediction would you make concerning their standard molar entropies at 298 K?
Oa. Even if more conditions are specified, a reliable prediction cannot be made.
Ob. S
< S°
nitrogen trifluoride
ammonia
Oc.S
> S°
nitrogen trifluoride
ammonia
Od. S°
ammonia
nitrogen trifluonide
Oe. Other conditions need to be specified before a reliable prediction can be made.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

