On the Excel spreadsheet (available in the Lab folder on HuskyCT), enter the mass of KNO3 used and the volume of the solution for each trial, along with the temperature at which the first crystals appear. You will need to complete the spreadsheet with the correct function for each calculation. For example, to convert the solution volume in mL to L (cells C6 and C7 in the spreadsheet), divide the values in C6 by 1000 (that is, in Cell C7, insert the formula =C6/1000). Do the same for the other calculations to convert to molarity, solve for Ksp, take the natural logarithm of the Ksp value, convert °C to K, etc. Finally, once all the data has been calculated, make a plot of In(Ksp) vs. 1/T. Insert a trendline and record the slope and y-intercept values to calculate the values for AG and AH. From these you can use the Gibbs- Helmholtz equation to calculate the values for AS.
On the Excel spreadsheet (available in the Lab folder on HuskyCT), enter the mass of KNO3 used and the volume of the solution for each trial, along with the temperature at which the first crystals appear. You will need to complete the spreadsheet with the correct function for each calculation. For example, to convert the solution volume in mL to L (cells C6 and C7 in the spreadsheet), divide the values in C6 by 1000 (that is, in Cell C7, insert the formula =C6/1000). Do the same for the other calculations to convert to molarity, solve for Ksp, take the natural logarithm of the Ksp value, convert °C to K, etc. Finally, once all the data has been calculated, make a plot of In(Ksp) vs. 1/T. Insert a trendline and record the slope and y-intercept values to calculate the values for AG and AH. From these you can use the Gibbs- Helmholtz equation to calculate the values for AS.
Chemistry by OpenStax (2015-05-04)
1st Edition
ISBN:9781938168390
Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Chapter11: Solutions And Colloids
Section: Chapter Questions
Problem 3E: When KNO3 is dissolved in water, the resulting solution is significantly colder than the water was...
Related questions
Question
Need help filling out the rest of the data
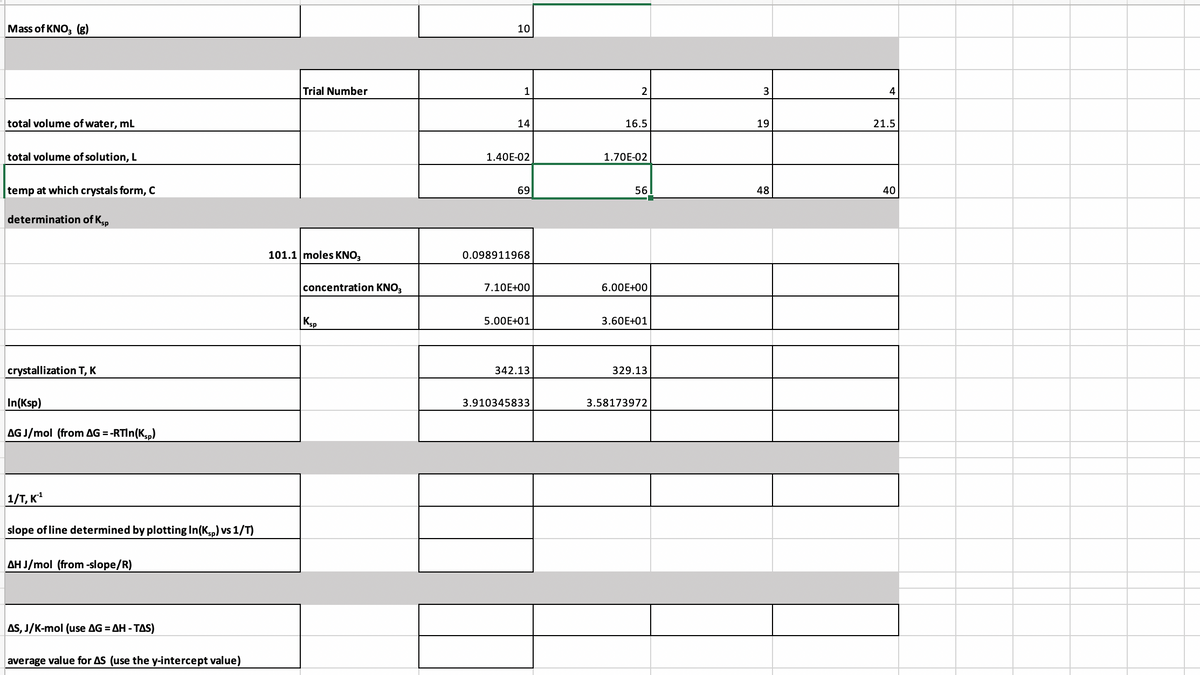
Transcribed Image Text:Mass of KNO, (g)
10
Trial Number
2
3
4
total volume of water, mL
14
16.5
19
21.5
total volume of solution, L
1.40E-02
1.70E-02
temp at which crystals form, C
69
56
48
40
determination of Kp
101.1 moles KNO3
0.098911968
concentration KNO,
7.10E+00
6.00E+00
Ksp
5.00E+01
3.60E+01
crystallization T, K
342.13
329.13
In(Ksp)
3.910345833
3.58173972
AGJ/mol (from AG =-RTIN(K,,)
1/T, K
slope of line determined by plotting In(Kp) vs 1/T)
AH J/mol (from -slope/R)
AS, J/K-mol (use AG = AH - TAS)
average value for AS (use the y-intercept value)
Transcribed Image Text:On the Excel spreadsheet (available in the Lab folder on HuskyCT), enter the mass of KNO3 used and the
volume of the solution for each trial, along with the temperature at which the first crystals appear. You
will need to complete the spreadsheet with the correct function for each calculation. For example, to
convert the solution volume in ml to L (cells C6 and C7 in the spreadsheet), divide the values in C6 by
1000 (that is, in Cell C7, insert the formula =C6/1000). Do the same for the other calculations to convert
to molarity, solve for Ksp, take the natural logarithm of the Ksp value, convert °C to K, etc. Finally, once
all the data has been calculated, make a plot of In(Ksp) vs. 1/T. Insert a trendline and record the slope
and y-intercept values to calculate the values for AG and AH. From these you can use the Gibbs-
Helmholtz equation to calculate the values for AS.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 5 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning