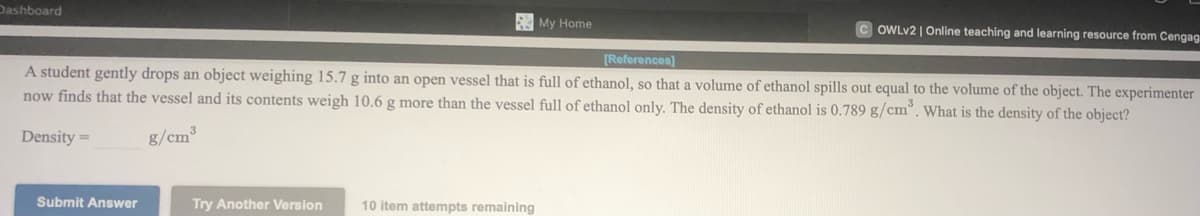
Online teaching and learning resource from Cenga [References] A student gently drops an object weighing 15.7 g into an open vessel that is full of ethanol, so that a volume of ethanol spills out equal to the volume of the object. The experimente now finds that the vessel and its contents weigh 10.6 g more than the vessel full of ethanol only. The density of ethanol is 0.789 g/cm, What is the density of the object? Density = g/cm Submit Answer Try Another Version 10 item attempts remaining
Online teaching and learning resource from Cenga [References] A student gently drops an object weighing 15.7 g into an open vessel that is full of ethanol, so that a volume of ethanol spills out equal to the volume of the object. The experimente now finds that the vessel and its contents weigh 10.6 g more than the vessel full of ethanol only. The density of ethanol is 0.789 g/cm, What is the density of the object? Density = g/cm Submit Answer Try Another Version 10 item attempts remaining
Chemistry: Principles and Reactions
8th Edition
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:William L. Masterton, Cecile N. Hurley
Chapter1: Matter And Measurements
Section: Chapter Questions
Problem 38QAP: A gasoline station in Manila, Philippines, charges 38.46 pesos per liter of unleaded gasoline at a...
Related questions
Question
100%
Transcribed Image Text:Dashboard
My Home
OWLV2 | Online teaching and learning resource from Cengag
[References)
A student gently drops an object weighing 15.7 g into an open vessel that is full of ethanol, so that a volume of ethanol spills out equal to the volume of the object. The experimenter
now finds that the vessel and its contents weigh 10.6 g more than the vessel full of ethanol only. The density of ethanol is 0.789 g/cm°. What is the density of the object?
Density =
g/cm³
Submit Answer
Try Another Version
10 item attempts remaining
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Recommended textbooks for you

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning
