only need help with the average molarity of KMnO4 solution (M) it must be a number. Reaction equation: 2 MnO4− + 5 C2O42− + 16 H+ → 2 Mn2+ + 10 CO2 + 8 H2O
only need help with the average molarity of KMnO4 solution (M) it must be a number. Reaction equation: 2 MnO4− + 5 C2O42− + 16 H+ → 2 Mn2+ + 10 CO2 + 8 H2O
Principles of Instrumental Analysis
7th Edition
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
ChapterA1: Evaluation Of Analytical Data
Section: Chapter Questions
Problem A1.22QAP
Related questions
Question
I only need help with the average molarity of KMnO4 solution (M) it must be a number.
Reaction equation:
2 MnO4− + 5 C2O42− + 16 H+ → 2 Mn2+ + 10 CO2 + 8 H2O
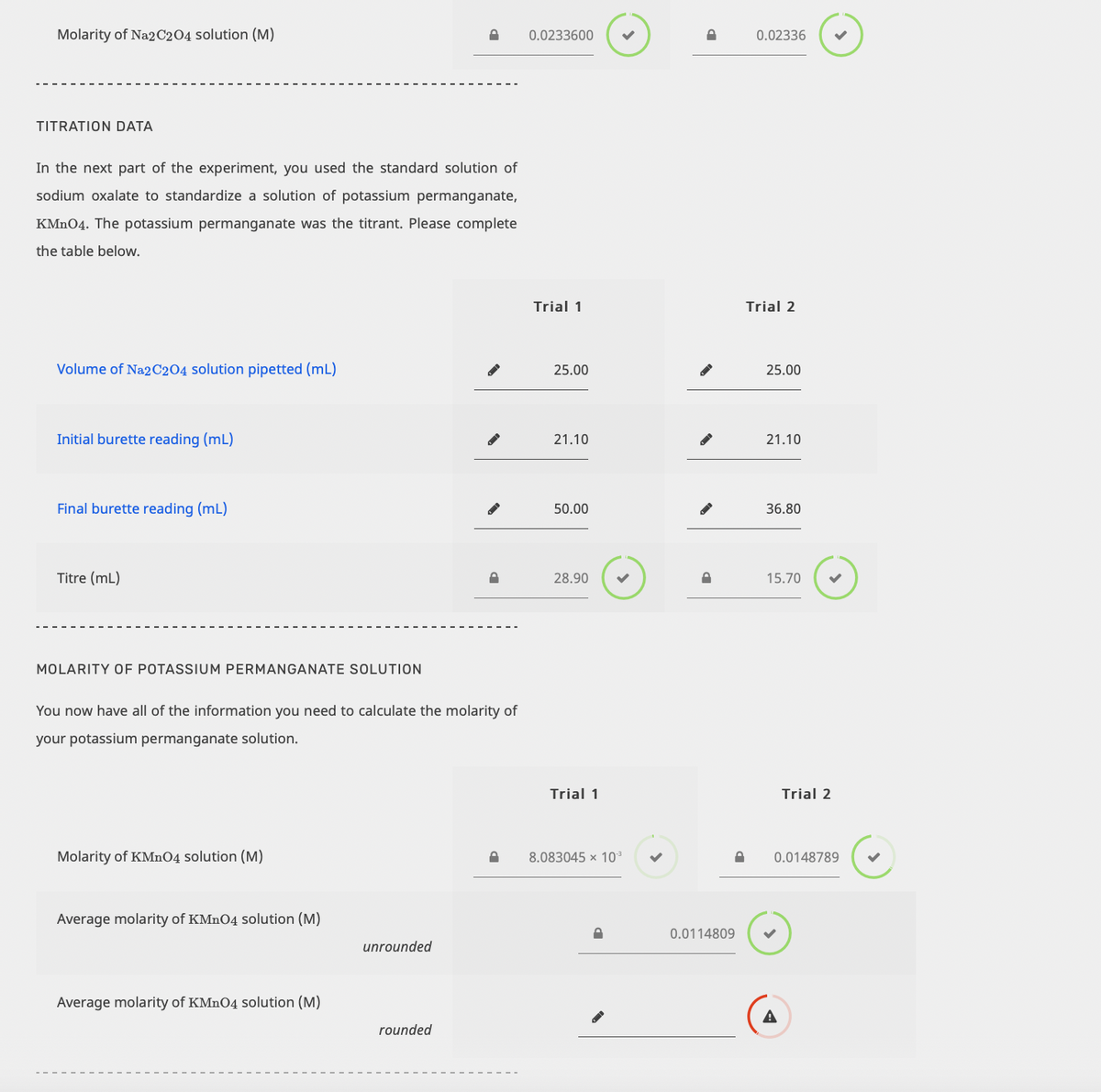
Transcribed Image Text:Molarity of Na2C204 solution (M)
0.0233600
0.02336
TITRATION DATA
In the next part of the experiment, you used the standard solution of
sodium oxalate to standardize a solution of potassium permanganate,
KMNO4. The potassium permanganate was the titrant. Please complete
the table below.
Trial 1
Trial 2
Volume of Na2C2O4 solution pipetted (mL)
25.00
25.00
Initial burette reading (mL)
21.10
21.10
Final burette reading (mL)
50.00
36.80
Titre (mL)
28.90
15.70
MOLARITY OF POTASSIUM PERMANGANATE SOLUTION
You now have all of the information you need to calculate the molarity of
your potassium permanganate solution.
Trial 1
Trial 2
Molarity of KMnO4 solution (M)
8.083045 x 103
0.0148789
Average molarity of KMNO4 solution (M)
0.0114809
unrounded
Average molarity of KMNO4 solution (M)
A
rounded
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

EBK A SMALL SCALE APPROACH TO ORGANIC L
Chemistry
ISBN:
9781305446021
Author:
Lampman
Publisher:
CENGAGE LEARNING - CONSIGNMENT


Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

EBK A SMALL SCALE APPROACH TO ORGANIC L
Chemistry
ISBN:
9781305446021
Author:
Lampman
Publisher:
CENGAGE LEARNING - CONSIGNMENT



Macroscale and Microscale Organic Experiments
Chemistry
ISBN:
9781305577190
Author:
Kenneth L. Williamson, Katherine M. Masters
Publisher:
Brooks Cole