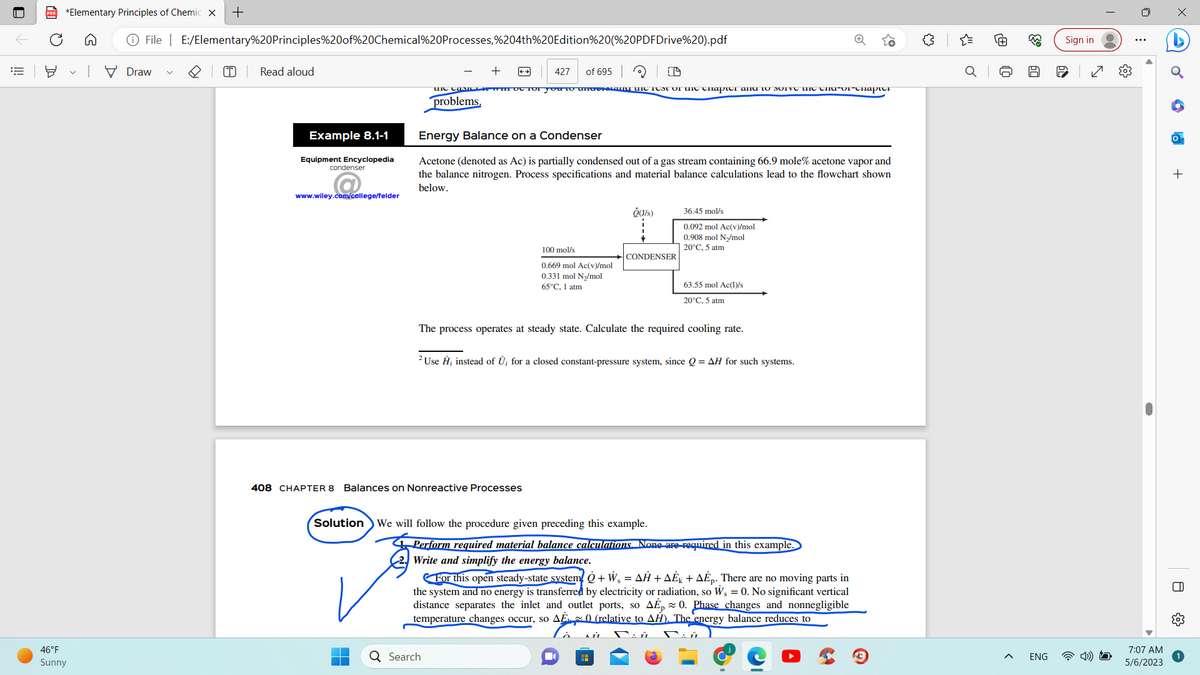
opedia ge/felder Acetone (denoted as Ac) is partially condensed out of a gas stream containing 66.9 mole% acetone vapor and the balance nitrogen. Process specifications and material balance calculations lead to the flowchart shown below. 100 mol/s 0.669 mol Ac(v/mol 0.331 mol Ny/mol 65°C. I am Quis) CONDENSER 36.45 mol/s 0.092 mol Ac(v)/mol 0.908 mol Ny/mol 20°C. 5 atm 63.55 mol Ac(lys 20°C, 5 atm The process operates at steady state. Calculate the required cooling rate.
opedia ge/felder Acetone (denoted as Ac) is partially condensed out of a gas stream containing 66.9 mole% acetone vapor and the balance nitrogen. Process specifications and material balance calculations lead to the flowchart shown below. 100 mol/s 0.669 mol Ac(v/mol 0.331 mol Ny/mol 65°C. I am Quis) CONDENSER 36.45 mol/s 0.092 mol Ac(v)/mol 0.908 mol Ny/mol 20°C. 5 atm 63.55 mol Ac(lys 20°C, 5 atm The process operates at steady state. Calculate the required cooling rate.
Introduction to Chemical Engineering Thermodynamics
8th Edition
ISBN:9781259696527
Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Chapter1: Introduction
Section: Chapter Questions
Problem 1.1P
Related questions
Question
I need help calculating enthalpies 2, 3 and 4.
Transcribed Image Text:||!
PDF *Elementary Principles of Chemic X +
46°F
Sunny
File | E:/Elementary%20Principles%20of%20Chemical%20Processes,%204th%20Edition%20(%20PDF Drive%20).pdf
Draw
(T) Read aloud
Example 8.1-1
Equipment Encyclopedia
condenser
www.wiley.com/college/felder
427 of 695
HA
Te Canice w UC for you to understand une rest of the chapter and to solve the cha-of-chapter
problems.
Energy Balance on a Condenser
Acetone (denoted as Ac) is partially condensed out of a gas stream containing 66.9 mole% acetone vapor and
the balance nitrogen. Process specifications and material balance calculations lead to the flowchart shown
below.
100 mol/s
0.669 mol Ac(v)/mol
0.331 mol N₂/mol
65°C, 1 atm
408 CHAPTER 8 Balances on Nonreactive Processes
Ô(J/s)
63.55 mol Ac(1)/s
20°C, 5 atm
The process operates at steady state. Calculate the required cooling rate.
Q Search
CONDENSER
2 Use Ĥ, instead of U; for a closed constant-pressure system, since Q=AH for such systems.
Solution We will follow the procedure given preceding this example.
36.45 mol/s
0.092 mol Ac(v)/mol
0.908 mol N₂/mol
20°C, 5 atm
■
Perform required material balance calculations. None are required in this example.
Write and simplify the energy balance.
For this open steady-state system + W, = AH + AÈk +AEp. There are no moving parts in
the system and no energy is transferred by electricity or radiation, so W, = 0. No significant vertical
distance separates the inlet and outlet ports, so AĖp ~0. Phase changes and nonnegligible
temperature changes occur, so AE 0 (relative to AH). The energy balance reduces to
r
P
<
Ơ
J
I
ENG
Sign in
100
7:07 AM
5/6/2023
•
la
+
O
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 1 images

Recommended textbooks for you

Introduction to Chemical Engineering Thermodynami…
Chemical Engineering
ISBN:
9781259696527
Author:
J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:
McGraw-Hill Education

Elementary Principles of Chemical Processes, Bind…
Chemical Engineering
ISBN:
9781118431221
Author:
Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:
WILEY

Elements of Chemical Reaction Engineering (5th Ed…
Chemical Engineering
ISBN:
9780133887518
Author:
H. Scott Fogler
Publisher:
Prentice Hall

Introduction to Chemical Engineering Thermodynami…
Chemical Engineering
ISBN:
9781259696527
Author:
J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:
McGraw-Hill Education

Elementary Principles of Chemical Processes, Bind…
Chemical Engineering
ISBN:
9781118431221
Author:
Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:
WILEY

Elements of Chemical Reaction Engineering (5th Ed…
Chemical Engineering
ISBN:
9780133887518
Author:
H. Scott Fogler
Publisher:
Prentice Hall


Industrial Plastics: Theory and Applications
Chemical Engineering
ISBN:
9781285061238
Author:
Lokensgard, Erik
Publisher:
Delmar Cengage Learning

Unit Operations of Chemical Engineering
Chemical Engineering
ISBN:
9780072848236
Author:
Warren McCabe, Julian C. Smith, Peter Harriott
Publisher:
McGraw-Hill Companies, The