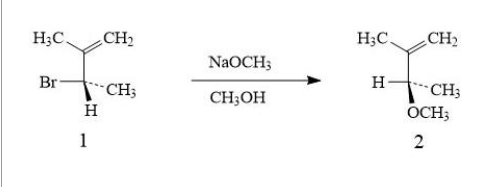
Optically pure Compound 1 undergoes a reaction at room temperature with sodium methoxide (NaOCH3) in methanol to form a single isomer of Compound 2 as shown below: 1. What are the stereochemical designations (R or S) of Compound 1 and Compound 2? 2. On the basis of the structure of Compound 2 and the information on the reaction conditions, suggest which type of mechanism Compound 1 undergoes . 3. The rate of the above reaction is determined experimentally to follow second-order kinetics. Give a fully labelled sketch of a reaction coordinate diagram for the reaction.
Optically pure Compound 1 undergoes a reaction at room temperature with sodium methoxide (NaOCH3) in methanol to form a single isomer of Compound 2 as shown below:
1. What are the stereochemical designations (R or S) of Compound 1 and Compound 2?
2. On the basis of the structure of Compound 2 and the information on the reaction conditions, suggest which type of mechanism Compound 1 undergoes .
3. The rate of the above reaction is determined experimentally to follow second-order kinetics. Give a fully labelled sketch of a reaction coordinate diagram for the reaction.
4. draw a mechanism on a piece of paper (using curly arrows) to show the formation of Compound 2 from Compound 1 including any activated complex.
5. If the sodium methoxide is left out of the reaction mixture, Compound 2 is formed in roughly equal amounts with another compound (Compound 3). Suggest a structure for Compound 3. With reference to the mechanism of the reaction and the structure of Compound 1, explain how these two Compounds 2 and 3 are formed under these conditions
Trending now
This is a popular solution!
Step by step
Solved in 5 steps with 6 images


