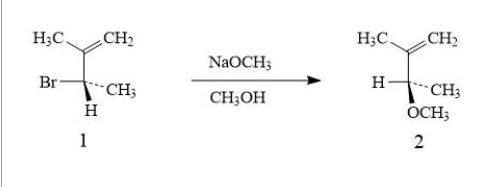
Optically pure Compound 1 undergoes a reaction at room temperature with sodium methoxide (NaOCH3) in methanol to form a single isomer of Compound 2 as shown below: 3. The rate of the above reaction is determined experimentally to follow second-order kinetics. Give a fully labelled sketch of a reaction coordinate diagram for the reaction. 4. draw a mechanism on a piece of paper (using curly arrows) to show the formation of Compound 2 from Compound 1 including any activated complex.
Reactions of Alkyl and Aryl halides
In organic chemistry, an alkyl halide is formed when an atom of hydrogen is switched by a halogen in a hydrocarbon or aliphatic compound. An aryl halide is formed when an atom of hydrogen is substituted by a halogen atom in an aromatic compound. Metals react with aryl halides and alkyl halides and they also go through nucleophilic substitution reactions and elimination reactions.
Zaitsev's Rule in Organic Chemistry
Alexander Zaitsev (also pronounced as Saytzeff), in 1875, prepared a rule to help predict the result of elimination reactions which stated, "The favored product in dehydrohalogenation reactions is that alkene that has the majority of alkyl groups attached to the double-bonded carbon atoms."
Tosylate
Tosylates are important functional groups in organic chemistry, mainly because of two important properties which they possess:
Alkyl Halides
A functional group is a collection of several atoms or bonds with certain characteristic chemical properties and reactions associated with it. There is a presence of a halogen atom (F, Cl, Br, or I; it represents any halogen atom), as a functional group in alkyl halides. Therefore, it can be said that alkanes that contain a halogen compound are called alkyl halides.
Optically pure Compound 1 undergoes a reaction at room temperature with sodium methoxide (NaOCH3) in methanol to form a single isomer of Compound 2 as shown below:
3. The rate of the above reaction is determined experimentally to follow second-order kinetics. Give a fully labelled sketch of a reaction coordinate diagram for the reaction.
4. draw a mechanism on a piece of paper (using curly arrows) to show the formation of Compound 2 from Compound 1 including any activated complex.
Step by step
Solved in 3 steps with 2 images


