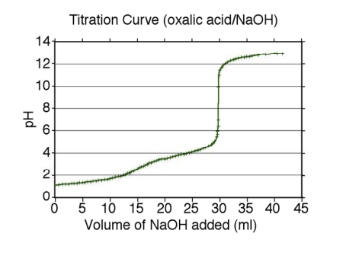
Oxalic acid (H2C2O4) is an acid widely used in chemical analysis, industry and veterinary applications. THE Neutralization volumetry is one of the options for determining this acid in raw materials for the industry. Considering this determination, answer the items below. Being that 0.2255 g of a sample of oxalic acid raw material was weighed and titrated completely with 23.2 mL of a 0.1522 mol/L NaOH solution, what is the percent purity of this product? (Pasta molecular oxalic acid 90.03 g mol/L) [Answer; purity = 70.5%] H2C2O4 (aq) + NaOH (aq) → Na2C2O4 (aq) + H2O(l) And suggest an appropriate indicator for the titration of this acid by looking at the titration curve profile below (photo)
Oxalic acid (H2C2O4) is an acid widely used in chemical analysis, industry and veterinary applications. THE Neutralization volumetry is one of the options for determining this acid in raw materials for the industry. Considering this determination, answer the items below. Being that 0.2255 g of a sample of oxalic acid raw material was weighed and titrated completely with 23.2 mL of a 0.1522 mol/L NaOH solution, what is the percent purity of this product? (Pasta molecular oxalic acid 90.03 g mol/L) [Answer; purity = 70.5%] H2C2O4 (aq) + NaOH (aq) → Na2C2O4 (aq) + H2O(l) And suggest an appropriate indicator for the titration of this acid by looking at the titration curve profile below (photo)
Chapter15: Complex Acid/base Systems
Section: Chapter Questions
Problem 15.27QAP
Related questions
Question
Oxalic acid (H2C2O4) is an acid widely used in chemical analysis, industry and veterinary applications. THE Neutralization volumetry is one of the options for determining this acid in raw materials for the industry. Considering this determination, answer the items below.
Being that 0.2255 g of a sample of oxalic acid raw material was weighed and titrated completely with 23.2 mL of a 0.1522 mol/L NaOH solution, what is the percent purity of this product? (Pasta molecular oxalic acid 90.03 g mol/L) [Answer; purity = 70.5%]
H2C2O4 (aq) + NaOH (aq) → Na2C2O4 (aq) + H2O(l)
And suggest an appropriate indicator for the titration of this acid by looking at the titration curve profile below (photo)
Transcribed Image Text:Hd
141
12-
10
8
6-
4.
2-
0
Titration Curve (oxalic acid/NaOH)
5 10 15 20 25 30 35 40 45
Volume of NaOH added (ml)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 7 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you





