P7E.1 If the vibration of a diatomic A-B is modelled using a harmonic oscillator, the vibrational frequency is given by w=(k; /u)", where u is the effective mass, µ=m,m,/(m, +m,). If atom A is substituted by an isotope (for example H substituted for 'H), then to a good approximation the force constant remains the same. Why? (Hint: Is there any change in the number of charged species?) (a) Show that when an isotopic substitution is made for atom A, such that its mass changes from m, to m, the vibrational frequency of A'-B, wAp can be expressed in terms of the vibrational frequency of A-B, @ An as o x=0 A (H/H", where u AB and u, are the effective masses of A-B and A'-B, respectively. (b) The vibrational frequency of 'HCl is 5.63 x 10"s". Calculate the vibrational frequency of (i) 'H*Cl and (ii) 'H"Cl. Use integer relative atomic masses. A'B
P7E.1 If the vibration of a diatomic A-B is modelled using a harmonic oscillator, the vibrational frequency is given by w=(k; /u)", where u is the effective mass, µ=m,m,/(m, +m,). If atom A is substituted by an isotope (for example H substituted for 'H), then to a good approximation the force constant remains the same. Why? (Hint: Is there any change in the number of charged species?) (a) Show that when an isotopic substitution is made for atom A, such that its mass changes from m, to m, the vibrational frequency of A'-B, wAp can be expressed in terms of the vibrational frequency of A-B, @ An as o x=0 A (H/H", where u AB and u, are the effective masses of A-B and A'-B, respectively. (b) The vibrational frequency of 'HCl is 5.63 x 10"s". Calculate the vibrational frequency of (i) 'H*Cl and (ii) 'H"Cl. Use integer relative atomic masses. A'B
Principles of Instrumental Analysis
7th Edition
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Chapter18: Raman Spectroscopy
Section: Chapter Questions
Problem 18.5QAP
Related questions
Question
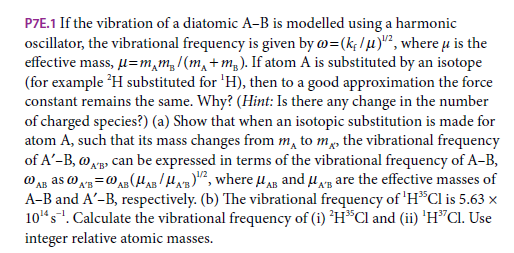
Transcribed Image Text:P7E.1 If the vibration of a diatomic A-B is modelled using a harmonic
oscillator, the vibrational frequency is given by w=(k; /u)", where u is the
effective mass, µ=m,m,/(m, +m,). If atom A is substituted by an isotope
(for example H substituted for 'H), then to a good approximation the force
constant remains the same. Why? (Hint: Is there any change in the number
of charged species?) (a) Show that when an isotopic substitution is made for
atom A, such that its mass changes from m, to m, the vibrational frequency
of A'-B, wAp can be expressed in terms of the vibrational frequency of A-B,
@ An as o x=0 A (H/H", where u AB and u, are the effective masses of
A-B and A'-B, respectively. (b) The vibrational frequency of 'HCl is 5.63 x
10"s". Calculate the vibrational frequency of (i) 'H*Cl and (ii) 'H"Cl. Use
integer relative atomic masses.
A'B
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 6 steps with 4 images

Recommended textbooks for you

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning