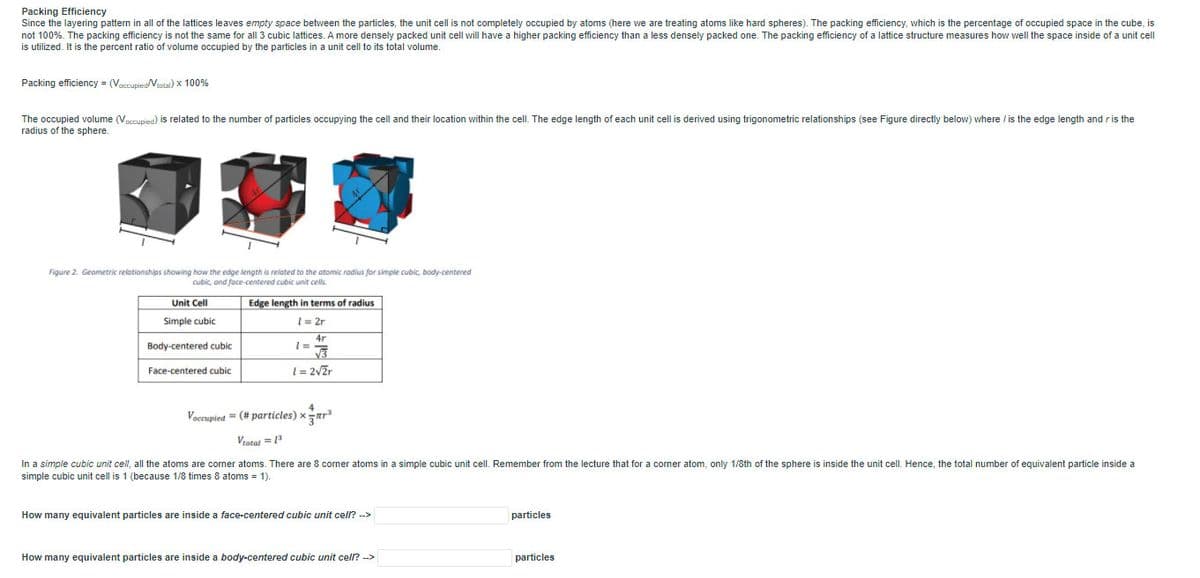
Packing Efficiency Since the layering pattern in all of the lattices leaves empty space between the particles, the unit cell is not completely occupied by atoms (here we are treating atoms like hard spheres). The packing efficiency, which is the percentage of occupied space in the cube, is not 100%. The packing efficiency is not the same for all 3 cubic lattices. A more densely packed unit cell will have a higher packing efficiency than a less densely packed one. The packing efficiency of a lattice structure measures how well the space inside of a unit cell is utilized. It is the percent ratio of volume occupied by the particles in a unit cell to its total volume. Packing efficiency (Voctupieotal) x 100% The occupied volume (Vacnupied) is related to the number of particles occupying the cell and their location within the cell. The edge length of each unit cell is derived using trigonometric relationships (see Figure directly below) where / is the edge length and ris the radius of the sphere. Figure 2. Geametric relationships showing how the edge length is related to the atomic radius for simple cubic, body-centered Cubic, and face-centered cubic unit cels Unit Cell Edge length terms of radius Simple cubic 1= 2r 4r Body-centered cubic Face-centered cubic 1= 2vZr Voccupied = (# particles) xar Vrotat = In a simple cubic unit ceil, all the atoms are corner atoms. There are 8 corner atoms in a simple cubic unit cell. Remember from the lecture that for a corner atom, only 1/8th of the sphere is inside the unit celL. Hence, the total number of equivalent particle inside a simple cubic unit cell is 1 (because 1/8 times 8 atoms = 1). How many equivalent particles are inside a face-centered cubic unit cell? -> particles How many equivalent particles are inside a body-centered cubic unit cell? -> particles
Packing Efficiency Since the layering pattern in all of the lattices leaves empty space between the particles, the unit cell is not completely occupied by atoms (here we are treating atoms like hard spheres). The packing efficiency, which is the percentage of occupied space in the cube, is not 100%. The packing efficiency is not the same for all 3 cubic lattices. A more densely packed unit cell will have a higher packing efficiency than a less densely packed one. The packing efficiency of a lattice structure measures how well the space inside of a unit cell is utilized. It is the percent ratio of volume occupied by the particles in a unit cell to its total volume. Packing efficiency (Voctupieotal) x 100% The occupied volume (Vacnupied) is related to the number of particles occupying the cell and their location within the cell. The edge length of each unit cell is derived using trigonometric relationships (see Figure directly below) where / is the edge length and ris the radius of the sphere. Figure 2. Geametric relationships showing how the edge length is related to the atomic radius for simple cubic, body-centered Cubic, and face-centered cubic unit cels Unit Cell Edge length terms of radius Simple cubic 1= 2r 4r Body-centered cubic Face-centered cubic 1= 2vZr Voccupied = (# particles) xar Vrotat = In a simple cubic unit ceil, all the atoms are corner atoms. There are 8 corner atoms in a simple cubic unit cell. Remember from the lecture that for a corner atom, only 1/8th of the sphere is inside the unit celL. Hence, the total number of equivalent particle inside a simple cubic unit cell is 1 (because 1/8 times 8 atoms = 1). How many equivalent particles are inside a face-centered cubic unit cell? -> particles How many equivalent particles are inside a body-centered cubic unit cell? -> particles
Chemistry: The Molecular Science
5th Edition
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:John W. Moore, Conrad L. Stanitski
Chapter9: Liquids, Solids, And Materials
Section: Chapter Questions
Problem 115QRT
Related questions
Question
Transcribed Image Text:Packing Efficiency
Since the layering pattern in all of the lattices leaves empty space between the particles, the unit cell is not completely occupied by atoms (here we are treating atoms like hard spheres). The packing efficiency, which is the percentage of occupied space in the cube, is
not 100%. The packing efficiency is not the same for all 3 cubic lattices. A more densely packed unit cell will have a higher packing efficiency than a less densely packed one. The packing efficiency of a lattice structure measures hovw well the space inside of a unit cell
is utilized. It is the percent ratio of volume occupied by the particles in a unit cell to its total volume.
Packing efficiency (VaceupiesVptal) x 100%
particles occupying the cell and their location within the cell. The edge length of each unit cell is derived using trigonometric relationships (see Figure directly below) where / is the edge length and ris the
The occupied volume (Veccupied) is related to the number
radius of the sphere.
Figure 2. Geometric relationships showing how the edge length is related to the atomic radius for simple cubic, body-centered
cubic, and face-centered cubic unitr cells.
Unit Cell
Edge length
terms of radius
Simple cubic
1= 2r
4r
Body-centered cubic
1= 2vZr
Face-centered cubic
Voccupied = (# particles) xar
Vtotat = 13
In a simple cubic unit cell, all the atoms are corner atoms. There are 8 corner atoms in a simple cubic unit cell. Remember from the lecture that for a corner atom, only 1/8th of the sphere is inside the unit cell. Hence, the total number of equivalent particle inside a
simple cubic unit cell is 1 (because 1/8 times 8 atoms = 1).
How many equivalent particles are inside a face-centered cubic unit cell? -->
particles
How many equivalent particles are inside a body-centered cubic unit cell? -->
particles
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemical Principles in the Laboratory
Chemistry
ISBN:
9781305264434
Author:
Emil Slowinski, Wayne C. Wolsey, Robert Rossi
Publisher:
Brooks Cole