panone (acetone) The structure of the alkene D CH CIHCH CHCIL CH: O (CH)C-C(CHH): O (CH:)CCH=CH O CH:CH;CH=C(CH; The condensation of 6-aminohexanoic acid, u he used to prepare a
panone (acetone) The structure of the alkene D CH CIHCH CHCIL CH: O (CH)C-C(CHH): O (CH:)CCH=CH O CH:CH;CH=C(CH; The condensation of 6-aminohexanoic acid, u he used to prepare a
Organic Chemistry
8th Edition
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Chapter11: Ethers, Epoxides, And Sulfides
Section: Chapter Questions
Problem 11.37P: Following are the steps in the industrial synthesis of glycerin. Provide structures for all...
Related questions
Question
Answer Q47, 48
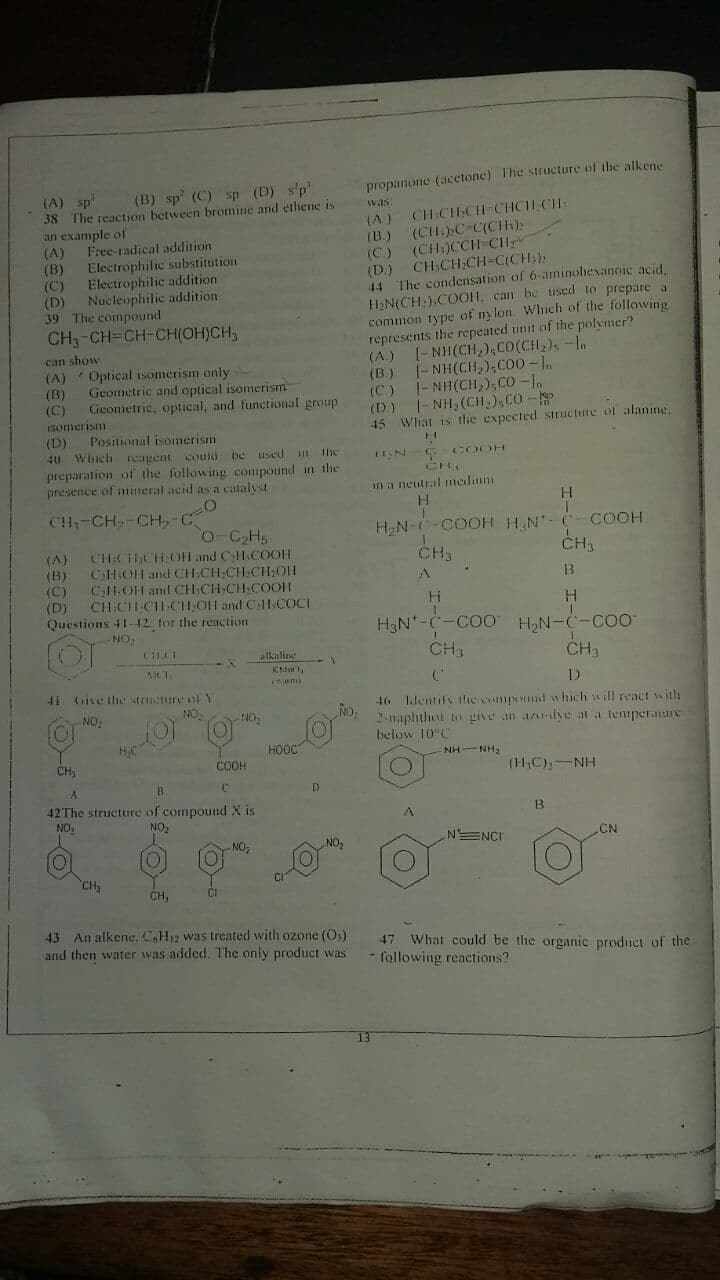
Transcribed Image Text:propanone (acetone) The structure of the alkene
(A) sp"
38 The reaction between bromine and ethene is
an example of
(A)
(B)
(B) sp' (C) sp (D) s'p
SPA
(A)
CH CCH CHCILCH:
(B.)
(CH:)CCH CHy
Free-radical addition
(CH).C-C(CIH):
(C.)
CH CH:CH-C(CH;):
Electrophilic substitution
(D.)
44 The condensation of 6-aminohexanoie acid,
HN(CH:).COO1, can be used to prepare a
common type of ny lon. Which of the following
represents the repeated unit of the polymer?
(- NH(CH,),CO(CH,), -In
(C)
Electrophilic addition
(D)
39 The compound
CH3-CH=CH-CH(OH)CH,
Nucleophilic addition
can show
(A.)
(A)
(B)
Optical isomerism only
Geometric and optical isomerism
|- NH(CH,),C00 - n
(B.)
(C)
|- NH(CH,), CO -ln
Geometric, optical, and functional group
(C)
Isomerism
(D)
40 Which reagent couid be uscd
preparation of the following compound in the
presence of mineral acid as a catalvst
|- NH, (CH,),CO -
(D.)
45 Wliat is the expected structure of alanine.
Positional isomerism
COOH
in a neutral nmedium
H.
CH,-CH,-CH,-C
H,N--COOH H N- C- COOH
CH3
(A)
(B)
(C)
O CHs
CHCHLCHOH and CHCOOH
CHOH and CH.CH CH CH,OH
C1-OH and CH CH CH COOH
CH CHCIH-CHOH and ClHCOCI
CH3
13
(D)
Questions 41-12 tor the reaction
H.
H3N-C-CO0 H,N-C-COO
NO.
CH3
alkaline
CH3
D
41 Give the Nr re olY
46 Identily the compound which will react with
2-naphthot to give an azodyeat a temperature
NO:
NO.
NO
below 10°C
HOOC
NH-NH
CH;
COOH
(H,C),-NH
D.
42The structure of compound X is
NO,
NO,
-NO,
NO
N NCI
CN
CH
CH
43 An alkene, C.H12 was treated with ozone (O)
47 What could be the organic produci of the
- following reactions?
and then water was added. The only product was
13
![48 Select the strongest acid from the following:
FCH,CO;H: CICH CO-H: BRCH CO:H
ICH,CO,l is
FCH CO:H
CICH;CO.H
BRCH2CO;H
ICH,CO,H
Br/NAOH
CH,CONH,
Heat
(A)
(B)
(A.)
(В)
(C.)
(D.)
CH:COBr
CH,CH2NH,
CH CONHBr
CHINII:
(C)
(D)
DIRECTIONS. Each of the following questions consists of a statement in the left-hand column followed
by a second statement in the right land column
Decide whether the first statement is true or false
Decide whether the second statement is true or false
Then on the answer sheet mark:
If both statements are true and the second statement is the correct explanation of the first statement
If both statemments are true but the second statement is NOT a correct explanation of the first
B.
statement
If the first stateinent is true and the second statement is false
If the first statement is false but the second statement is true
DIRECTIONS SUMMARY
First Statement
Second Statement
Second statement is a correct explanation of the first
Second statement is NOT a corect explanation of the first
True
True
True
True
True
False
D
False
True
First statement
Second statement
P'henylethanone (acetophenone) gives a phenylethanone (pheny lacetone) contain the
-COCH group
Aniline (pheny lamine) reacts with ice- Phenylamine (Aniine) is a primary aromatic
49.
positive iodoform test
50.
cold nitrous acii liherating nitrogen gas and amine
forming phenol
SET 3: MCQ (CGCEB 2011).
moles of water
Which of the following elements is most
likely to have the following ionisation energies
(kJmol")? 780, 1580, 3230, 4360, 16000
(A] Aluminium (B] Silicon
[C] Magnesium (D] Phosphorus
Question
experiment.
Ah vitr :
place in a test tube and 5 cm (0.05 mole) of
hydrochloric acid was added. On shaking the test
tube, the solid dissolved. On adding 2 cm' of
1.
How
of
3.
many
crystallization are there in 0.2 mole of sodium
sulphate Na, SO,. 10H,0?
(A) 0.2 (B) 2 (C) S (D) 20
2.
In which of the following equations does
the oxidation state (number) of the determined
Hom undereo the greatest change
4-7
concern
the
following
(A)
(B)
Ba0 + So, Baso,
Cl, + 2MN0, - 2MN0, + 201
(C)
(D)
2FECI, + SnCl, 2FECI, + SnCl,
14](/v2/_next/image?url=https%3A%2F%2Fcontent.bartleby.com%2Fqna-images%2Fquestion%2F5a6d9c67-6f13-49d2-ac4d-2d996f90a88b%2F503d30df-1dd7-4e0e-9695-16fdded664e6%2F3oitsv_processed.jpeg&w=3840&q=75)
Transcribed Image Text:48 Select the strongest acid from the following:
FCH,CO;H: CICH CO-H: BRCH CO:H
ICH,CO,l is
FCH CO:H
CICH;CO.H
BRCH2CO;H
ICH,CO,H
Br/NAOH
CH,CONH,
Heat
(A)
(B)
(A.)
(В)
(C.)
(D.)
CH:COBr
CH,CH2NH,
CH CONHBr
CHINII:
(C)
(D)
DIRECTIONS. Each of the following questions consists of a statement in the left-hand column followed
by a second statement in the right land column
Decide whether the first statement is true or false
Decide whether the second statement is true or false
Then on the answer sheet mark:
If both statements are true and the second statement is the correct explanation of the first statement
If both statemments are true but the second statement is NOT a correct explanation of the first
B.
statement
If the first stateinent is true and the second statement is false
If the first statement is false but the second statement is true
DIRECTIONS SUMMARY
First Statement
Second Statement
Second statement is a correct explanation of the first
Second statement is NOT a corect explanation of the first
True
True
True
True
True
False
D
False
True
First statement
Second statement
P'henylethanone (acetophenone) gives a phenylethanone (pheny lacetone) contain the
-COCH group
Aniline (pheny lamine) reacts with ice- Phenylamine (Aniine) is a primary aromatic
49.
positive iodoform test
50.
cold nitrous acii liherating nitrogen gas and amine
forming phenol
SET 3: MCQ (CGCEB 2011).
moles of water
Which of the following elements is most
likely to have the following ionisation energies
(kJmol")? 780, 1580, 3230, 4360, 16000
(A] Aluminium (B] Silicon
[C] Magnesium (D] Phosphorus
Question
experiment.
Ah vitr :
place in a test tube and 5 cm (0.05 mole) of
hydrochloric acid was added. On shaking the test
tube, the solid dissolved. On adding 2 cm' of
1.
How
of
3.
many
crystallization are there in 0.2 mole of sodium
sulphate Na, SO,. 10H,0?
(A) 0.2 (B) 2 (C) S (D) 20
2.
In which of the following equations does
the oxidation state (number) of the determined
Hom undereo the greatest change
4-7
concern
the
following
(A)
(B)
Ba0 + So, Baso,
Cl, + 2MN0, - 2MN0, + 201
(C)
(D)
2FECI, + SnCl, 2FECI, + SnCl,
14
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning

Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning