Part 2: Mole Calculations 1. How many moles of sodium carbonate are there in 53.6 grams of Na2CO3? 2. How many moles of each atom are there in 33.3 moles of Fe3(PO4)2? 3. How many molecules of dinitrogen tetroxide (N204) are there in 59 g of N204? 4. How many grams does one molecule of carbon monoxide (CO) weigh? 5. How many oxygen gas molecules do you need to make 25 g of water vapor (H₂O)? Balanced chemical equation:
Part 2: Mole Calculations 1. How many moles of sodium carbonate are there in 53.6 grams of Na2CO3? 2. How many moles of each atom are there in 33.3 moles of Fe3(PO4)2? 3. How many molecules of dinitrogen tetroxide (N204) are there in 59 g of N204? 4. How many grams does one molecule of carbon monoxide (CO) weigh? 5. How many oxygen gas molecules do you need to make 25 g of water vapor (H₂O)? Balanced chemical equation:
Chemistry for Engineering Students
4th Edition
ISBN:9781337398909
Author:Lawrence S. Brown, Tom Holme
Publisher:Lawrence S. Brown, Tom Holme
Chapter3: Molecules, Moles, And Chemical Equations
Section: Chapter Questions
Problem 3.68PAE: 3.68 Magnesium is lighter than other structural metals, so it is increasingly important in the...
Related questions
Question
How can we complete part 2? With moles

Transcribed Image Text:Workshop 9: The Mole
The mole is the unit of measurement for an amount in chemistry and physics. It is defined as the
amount of sample that contains as many items as there are atoms in 12 grams of carbon-12 (¹2C
isotope). The number of items in 1 mole is called the Avogadro's number:
NA = 6.022141 x 1023 1/mol
Mole in science, just like a dozen in our everyday life, allows us to quickly count or compare large
(huge!) quantities of items (microscopic objects). And since mole is just a number - you can have
a mole of anything - atoms, molecules, ions, electrons, photons, etc.
For any substance, the number of grams in 1 mole is the same as the number of atomic mass
units in the molecular weight of the substance. The molar mass (or molar weight) of a chemical
substance is defined as the mass in grams of 1 mole of that substance. The units for molar mass
are grams per mole - g/mol.
The mole provides a bridge between the atom and the macroscopic amounts of material that we
work with in the laboratory or in the industrial setting. For example, it allows chemists to
determine how many grams of each of the reactants are needed to produce a sought number of
grams of product.
Part 1: Discussion of mole and mass
Discuss these questions among your group members: think about water molecules (H₂0) and
sugar molecules (C12H22011) and try to predict the answers in the table below. Just predict - do
not use calculator.
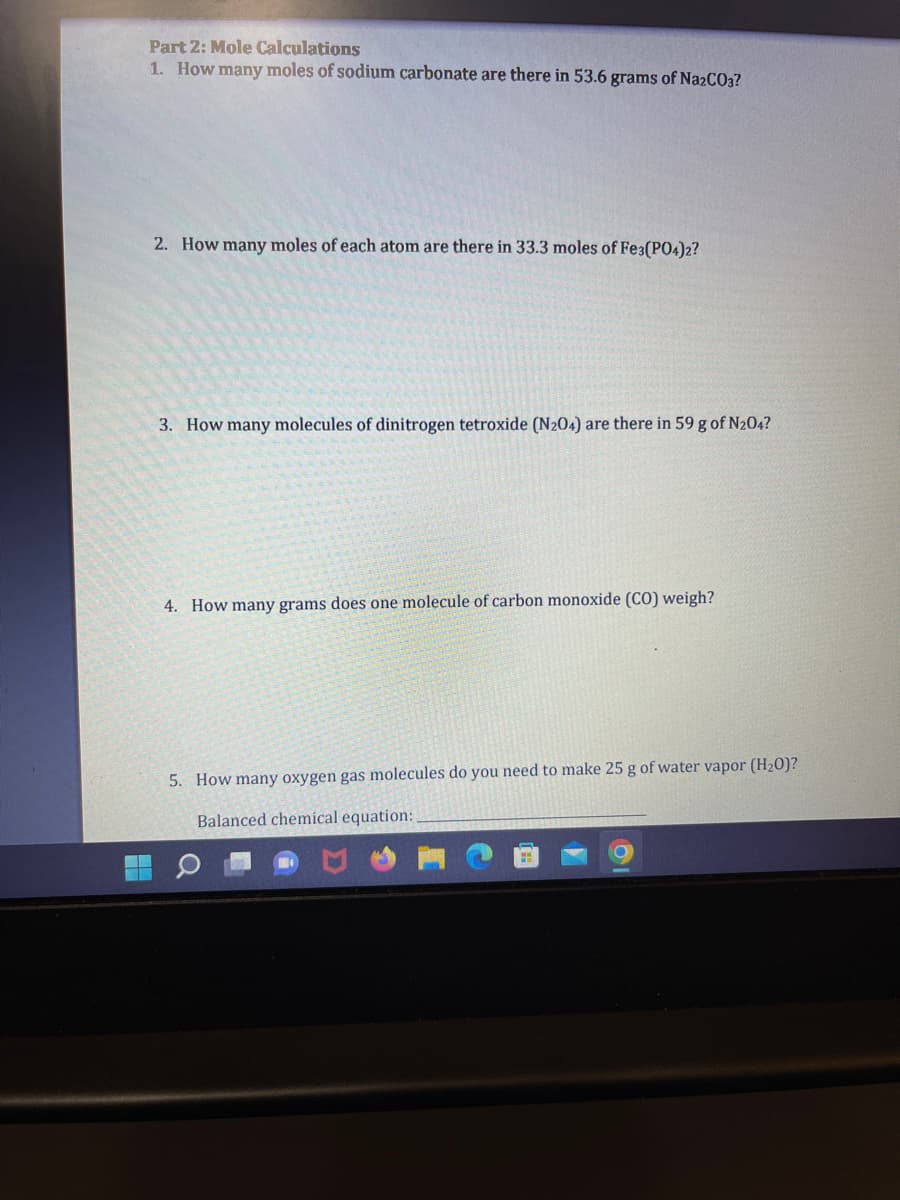
Transcribed Image Text:Part 2: Mole Calculations
1. How many moles of sodium carbonate are there in 53.6 grams of Na2CO3?
2. How many moles of each atom are there in 33.3 moles of Fe3(PO4)2?
3. How many molecules of dinitrogen tetroxide (N204) are there in 59 g of N₂04?
4. How many grams does one molecule of carbon monoxide (CO) weigh?
5. How many oxygen gas molecules do you need to make 25 g of water vapor (H₂0)?
Balanced chemical equation:
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning