Chapter32: Gas Chromatography
Section: Chapter Questions
Problem 32.22QAP
Related questions
Question
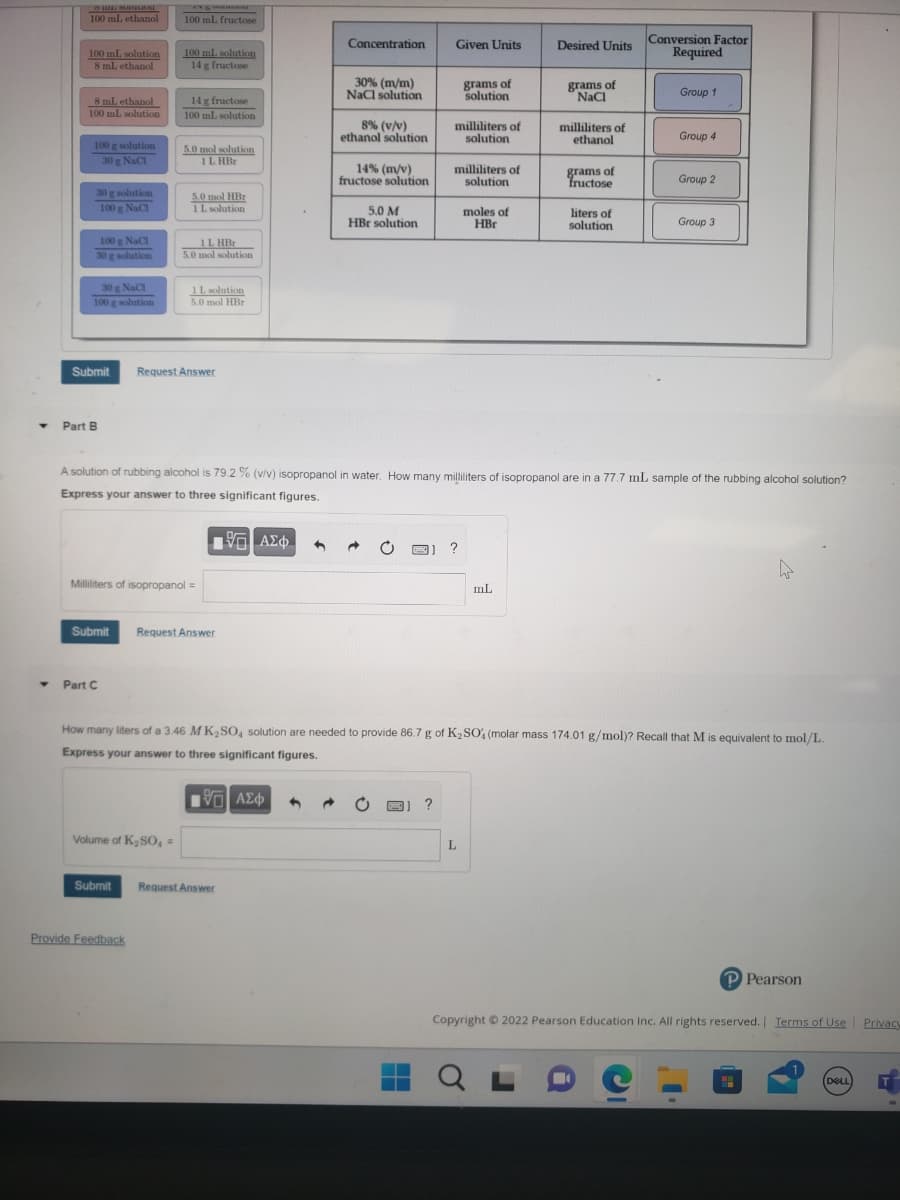
One question with three parts
Transcribed Image Text:Y
Y
HIE SORGERON
100 mL ethanol
100 mL solution
8 ml ethanol
8 mL ethanol
100 mL solution
100 g solution
30 g NaCl
30 g solution
100 g NaC1
100 g NaCl
30 g solution
30 g NaCl
100 g solution
Part B
Submit
100 ml. fructose
Part C
100 mL solution
14 g fructose
14 g fructose
100 mL solution
Submit Request Answer
5.0 mol solution
1 LHBr
Volume of K₂SO4 =
5.0 mol HBr
1 L solution
Provide Feedback
1 LHBr
5.0 mol solution
Milliliters of isopropanol =
1 L solution
5.0 mol HBr
Request Answer
Concentration
Submit Request Answer
30% (m/m)
NaCl solution
8% (v/v)
ethanol solution
14% (m/v)
fructose solution
5.0 M
HBr solution
Fak
Given Units
?
milliliters of
solution
grams of
solution
milliliters of
solution
?
moles of
HBr
A solution of rubbing alcohol is 79.2 % (v/v) isopropanol in water. How many milliliters of isopropanol are in a 77.7 mL sample of the rubbing alcohol solution?
Express your answer to three significant figures.
195| ΑΣΦ ←
L
mL
Desired Units
grams of
NaCl
milliliters of
ethanol
QL
grams of
fructose
liters of
solution
How many liters of a 3.46 MK₂SO4 solution are needed to provide 86.7 g of K₂SO4 (molar mass 174.01 g/mol)? Recall that M is equivalent to mol/L.
Express your answer to three significant figures.
195| ΑΣΦ 4
Conversion Factor
Required
Group 1
Group 4
-
Group 2
Group 3
Copyright © 2022 Pearson Education Inc. All rights reserved. | Terms of Use
P Pearson
DELL
Privacy
Transcribed Image Text:a mastering chemis X
der
M Reading Mode: 1.5... M Gmail
▾
Part A
F2
https://openvellum.ecollege.com/course.html?courseld=17485264&OpenVellumHMAC-567d4f4922241a0e1df5e0c226c1695a#1000
100 ml. ethanol
8 ml solution
8 ml solution
100
ml. ethanol
100 ml solution
8 mL ethanol
8 mL ethanol
100 ml solution
100 g solution
30 g NaCl
30 g solution
100 g NaC1
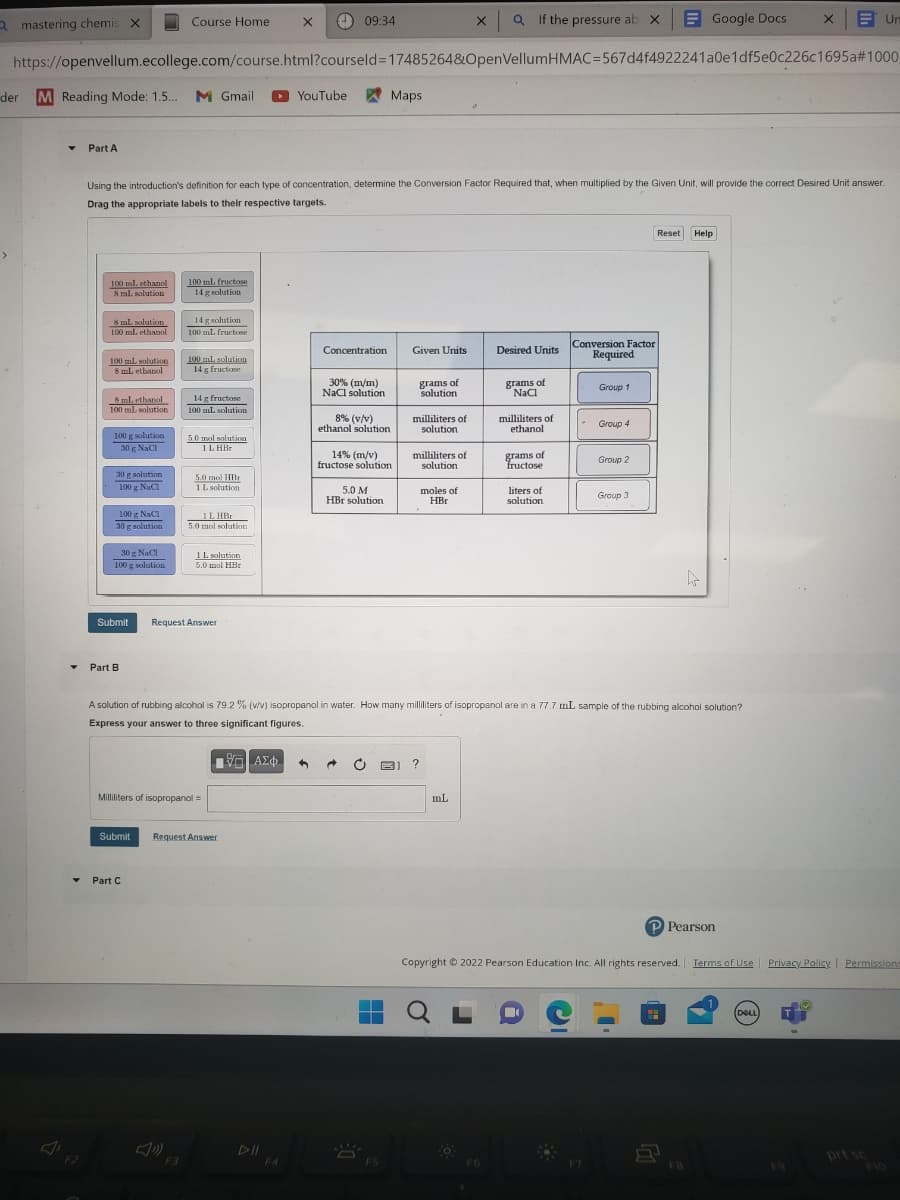
Using the introduction's definition for each type of concentration, determine the Conversion Factor Required that, when multiplied by the Given Unit, will provide the correct Desired Unit answer.
Drag the appropriate labels to their respective targets.
100 g NaCl
30 r solution
30 g solution
30g NaCl
100 g solution
Course Home. X
Submit
Part B
100 mL fructose
14 g solution
▼ Part C
14 g solution
100 mL fructose
100 mL solution
14 g fructose
14 g fructose
100 mL solution
5.0 mol solution
1 LHBr
5.0 mol HBr
1 L solution.
1L HBr
FO
5.0 mol solution
1 L solution
5.0 mol HBr
Request Answer
Milliliters of isopropanol =
Submit Request Answer
09:34
YouTube
F4
Concentration
30% (m/m)
NaCl solution
8% (v/v)
ethanol solution
14% (m/v)
fructose solution
Maps
5.0 M
HBr solution
Given Units
grams of
solution
milliliters of
solution
milliliters of
solution
moles of
HBr
?
X Q If the pressure ab X
mL
Q
Desired Units
grams of
NaCl
F6
milliliters of
ethanol
grams of
fructose
liters of
solution
A solution of rubbing alcohol is 79.2 % (v/v) isopropanol in water. How many milliliters of isopropanol are in a 77.7 mL sample of the rubbing alcohol solution?
Express your answer to three significant figures.
ΕΞΙ ΑΣΦ 4
Conversion Factor
Required
Group 1
-
Group 4
Group 2
Google Docs
Group 3
Reset Help
hr
P Pearson
Copyright © 2022 Pearson Education Inc. All rights reserved. Terms of Use Privacy Policy | Permissions
x
Un
DELL
9
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 6 steps with 11 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you



