Part 2 of 2 Atoms are spherical in shape. Therefore, the Pt atoms in the cube cannot fill all the available space. If only 74.0 percent of the space inside the cube is -22 g. [The volume of a sphere of radius taken up by Pt atoms, calculate the radius in picometers of a Pt atom. The mass of a single Pt atom is 3.240 x 10 .The volume of a cube is 13, where I is the length of a side. Avogadro's number is 6.022 x 1023. 4 of significant digits. ris 135.7 pm X S .] Be sure your answer has the correct number
Part 2 of 2 Atoms are spherical in shape. Therefore, the Pt atoms in the cube cannot fill all the available space. If only 74.0 percent of the space inside the cube is -22 g. [The volume of a sphere of radius taken up by Pt atoms, calculate the radius in picometers of a Pt atom. The mass of a single Pt atom is 3.240 x 10 .The volume of a cube is 13, where I is the length of a side. Avogadro's number is 6.022 x 1023. 4 of significant digits. ris 135.7 pm X S .] Be sure your answer has the correct number
Chemistry & Chemical Reactivity
10th Edition
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Chapter1: Basic Concepts Of Chemistry
Section: Chapter Questions
Problem 40RGQ: You need a cube of aluminum with a mass of 7.6 g. What must be the length of the cube's edge (in...
Related questions
Question
Pls help solve part 2 of the question, I have been trying to find the correct answer but everytime I get it wrong, pls pls make sure your answer is correct 10000% and explain pls and thank you sm
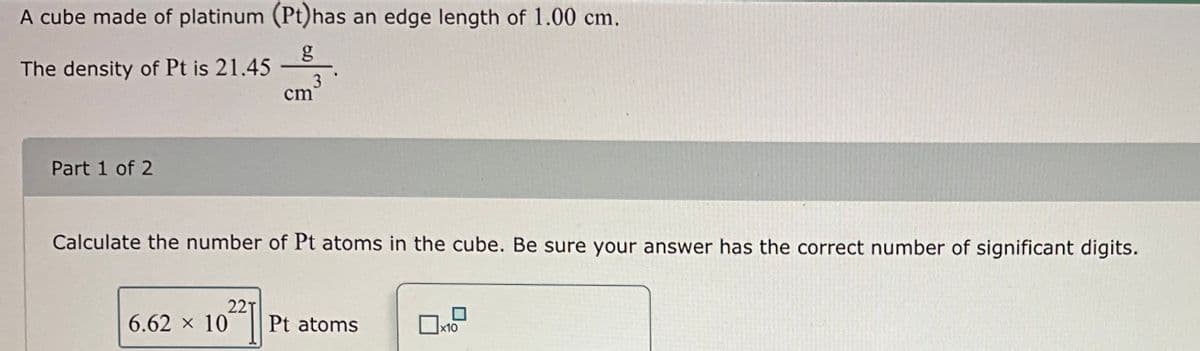
Transcribed Image Text:A cube made of platinum (Pt) has an edge length of 1.00 cm.
The density of Pt is 21.45
Part 1 of 2
02271
cm
Calculate the number of Pt atoms in the cube. Be sure your answer has the correct number of significant digits.
6.62 × 10
3
Pt atoms
x10
![Part 2 of 2
Atoms are spherical in shape. Therefore, the Pt atoms in the cube cannot fill all the available space. If only 74.0 percent of the space inside the cube is
g. [The volume of a sphere of radius
-22
taken up by Pt atoms, calculate the radius in picometers of a Pt atom. The mass of a single Pt atom is 3.240 x 10
4
³. The volume of a cube is 1³, where I is the length of a side. Avogadro's number is 6.022 × 1023 .] Be sure your answer has the correct number
3
of significant digits.
ris
X...
135.7 pm
Try one last time
x10
X
D](/v2/_next/image?url=https%3A%2F%2Fcontent.bartleby.com%2Fqna-images%2Fquestion%2F73a75771-70b1-4bb7-8045-da7c904db311%2F8fbd83f2-32d9-4758-ae60-b57b0713ee54%2F94ufwtc_processed.jpeg&w=3840&q=75)
Transcribed Image Text:Part 2 of 2
Atoms are spherical in shape. Therefore, the Pt atoms in the cube cannot fill all the available space. If only 74.0 percent of the space inside the cube is
g. [The volume of a sphere of radius
-22
taken up by Pt atoms, calculate the radius in picometers of a Pt atom. The mass of a single Pt atom is 3.240 x 10
4
³. The volume of a cube is 1³, where I is the length of a side. Avogadro's number is 6.022 × 1023 .] Be sure your answer has the correct number
3
of significant digits.
ris
X...
135.7 pm
Try one last time
x10
X
D
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 4 steps with 14 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning