Part 2. Writing formulas from chemical names Direction. Write the formula of the ions (including charges) expected from the following compound. POSITIVE ION NEGATIVE ION FORMULA COMPOUND Strontium chromate Calcium sulfate Tin (IV) oxide Sodium carbonate Potassium Chloride Lead lodide Bismuth nitrate Phosphorus pentachloride Calcium hydroxide Hydrochloric acid
Part 2. Writing formulas from chemical names Direction. Write the formula of the ions (including charges) expected from the following compound. POSITIVE ION NEGATIVE ION FORMULA COMPOUND Strontium chromate Calcium sulfate Tin (IV) oxide Sodium carbonate Potassium Chloride Lead lodide Bismuth nitrate Phosphorus pentachloride Calcium hydroxide Hydrochloric acid
Chemistry
10th Edition
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Chapter21: Transition Metals And Coordination Chemistry
Section: Chapter Questions
Problem 83AE
Related questions
Question
answer 6,7,8,9 and 10 row.
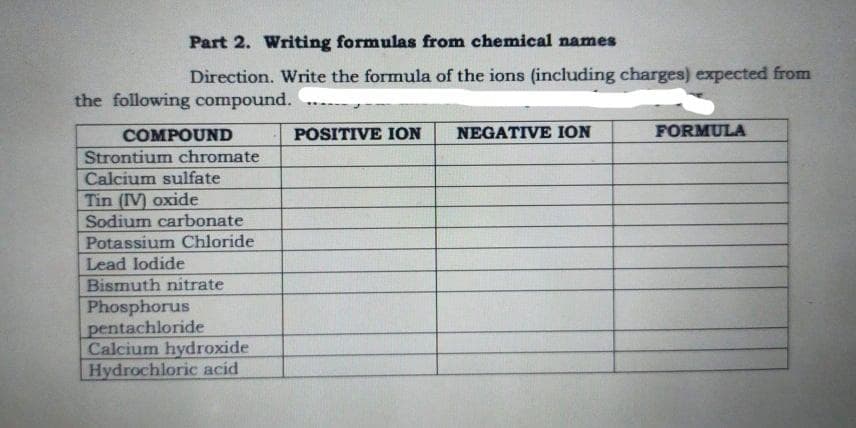
Transcribed Image Text:Part 2. Writing formulas from chemical names
Direction. Write the formula of the ions (including charges) expected from
the following compound.
POSITIVE ION
NEGATIVE ION
FORMULA
COMPOUND
Strontium chromate
Calcium sulfate
Tin (IV) oxide
Sodium carbonate
Potassium Chloride
Lead lodide
Bismuth nitrate
Phosphorus
pentachloride
Calcium hydroxide
Hydrochloric acid
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Recommended textbooks for you

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning