Part 5 X Your answer is incorrect. Try again. Using the answers from the previous parts, solve for the molality of the solution. 351 Use correct number of significant digits;
Part 5 X Your answer is incorrect. Try again. Using the answers from the previous parts, solve for the molality of the solution. 351 Use correct number of significant digits;
Chemistry: The Molecular Science
5th Edition
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:John W. Moore, Conrad L. Stanitski
Chapter13: The Chemistry Of Solutes And Solutions
Section: Chapter Questions
Problem 127QRT
Related questions
Question
I only need part 5, I have everything else.
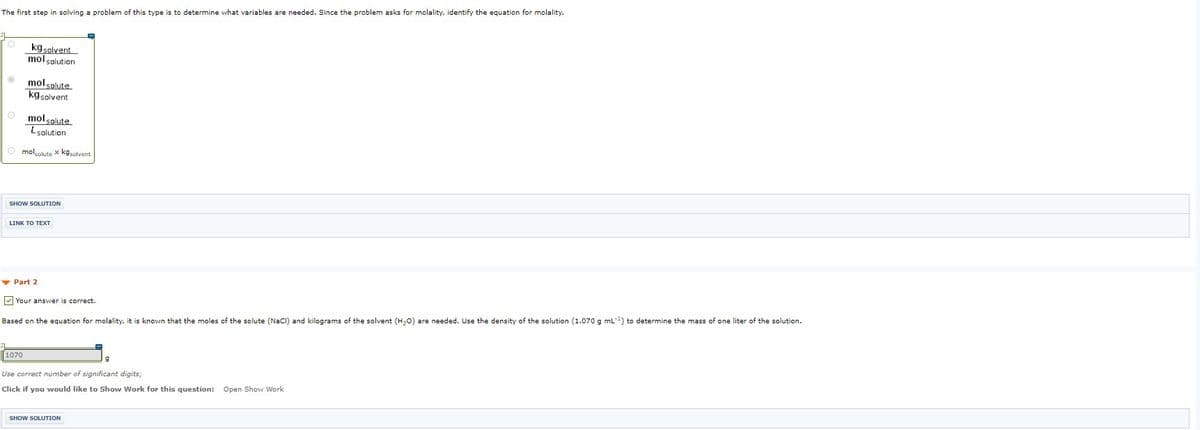
Transcribed Image Text:The first step in solving a problem of this type is to determine what variables are needed. Since the problem asks for molality, identify the equation for molality.
kg solvent
mol solution
mol solute
kgsolvent
O molsolute
Lsolution
O mololute x kgsolvent
SHOW SOLUTION
INK ΤOΤΕXT
v Part 2
V Your answer is correct.
Based on the equation for molality, it is known that the moles of the solute (NaCl) and kilograms of the solvent (H20) are needed. Use the density of the solution (1.070 g mL) to determine the mass of one liter of the solution.
1070
Use correct number of significant digits;
Open Show Work
Click if you would like to Show Work for this question:
SHOW SOLUTION
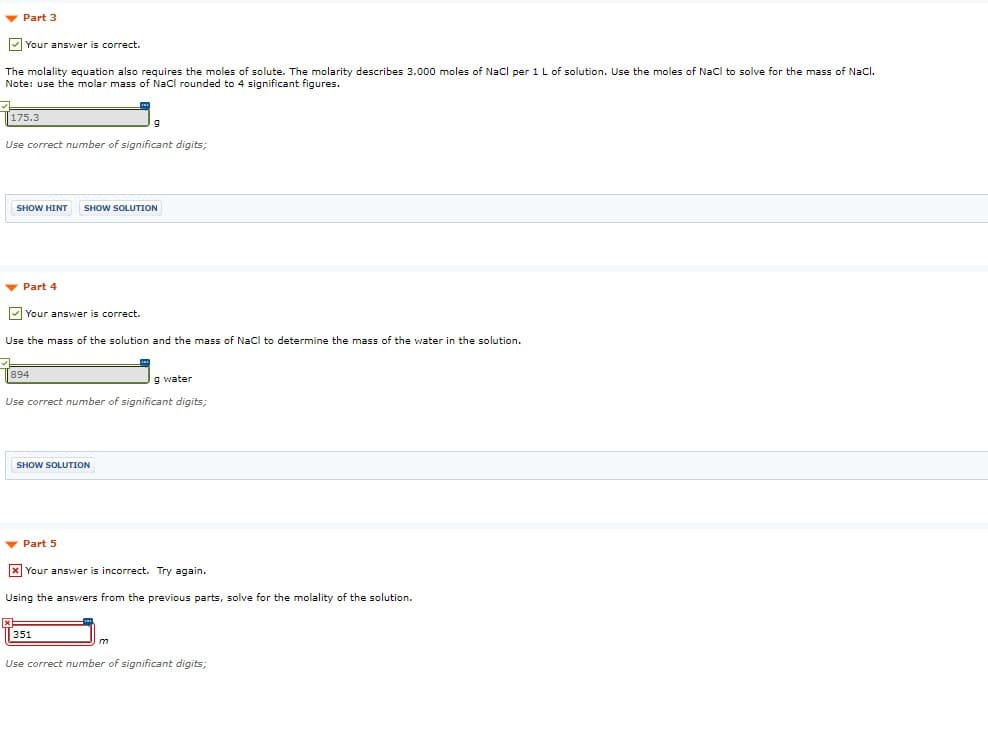
Transcribed Image Text:v Part 3
V Your answer is correct.
The molality equation also requires the moles of solute. The molarity describes 3.000 moles of Nacl per 1 L of solution. Use the moles of Nacl to solve for the mass of Nacl.
Note: use the molar mass of Nacl rounded to 4 significant figures.
175.3
Use correct number of significant digits;
SHOW HINT
SHOW SOLUTION
v Part 4
Your answer is correct.
Use the mass of the solution and the mass of Nacl to determine the mass of the water in the solution.
894
g water
Use correct number of significant digits;
SHOW SOLUTION
- Part 5
x Your answer is incorrect. Try again.
Using the answers from the previous parts, solve for the molality of the solution.
351
Use correct number of significant digits;
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

General, Organic, and Biological Chemistry
Chemistry
ISBN:
9781285853918
Author:
H. Stephen Stoker
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

General, Organic, and Biological Chemistry
Chemistry
ISBN:
9781285853918
Author:
H. Stephen Stoker
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning