Part A 5.0 g of nitrogen gas at 20° C and an initial pressure of 2.2 atm undergo a constant-pressure expansion until the volume has tripled. What is the gas volume after the expansion? Express your answer with the appropriate units. • View Available Hint(s) ol HA ? V = Value Units Submit Part B What is the gas temperature after the expansion? Express your answer in kelvins. • View Available Hint(s) ? T = K
Part A 5.0 g of nitrogen gas at 20° C and an initial pressure of 2.2 atm undergo a constant-pressure expansion until the volume has tripled. What is the gas volume after the expansion? Express your answer with the appropriate units. • View Available Hint(s) ol HA ? V = Value Units Submit Part B What is the gas temperature after the expansion? Express your answer in kelvins. • View Available Hint(s) ? T = K
College Physics
1st Edition
ISBN:9781938168000
Author:Paul Peter Urone, Roger Hinrichs
Publisher:Paul Peter Urone, Roger Hinrichs
Chapter13: Temperature, Kinetic Theory, And The Gas Laws
Section: Chapter Questions
Problem 4CQ: If you add boiling water to a cup at room temperature, what would you expect the final equilibrium...
Related questions
Question
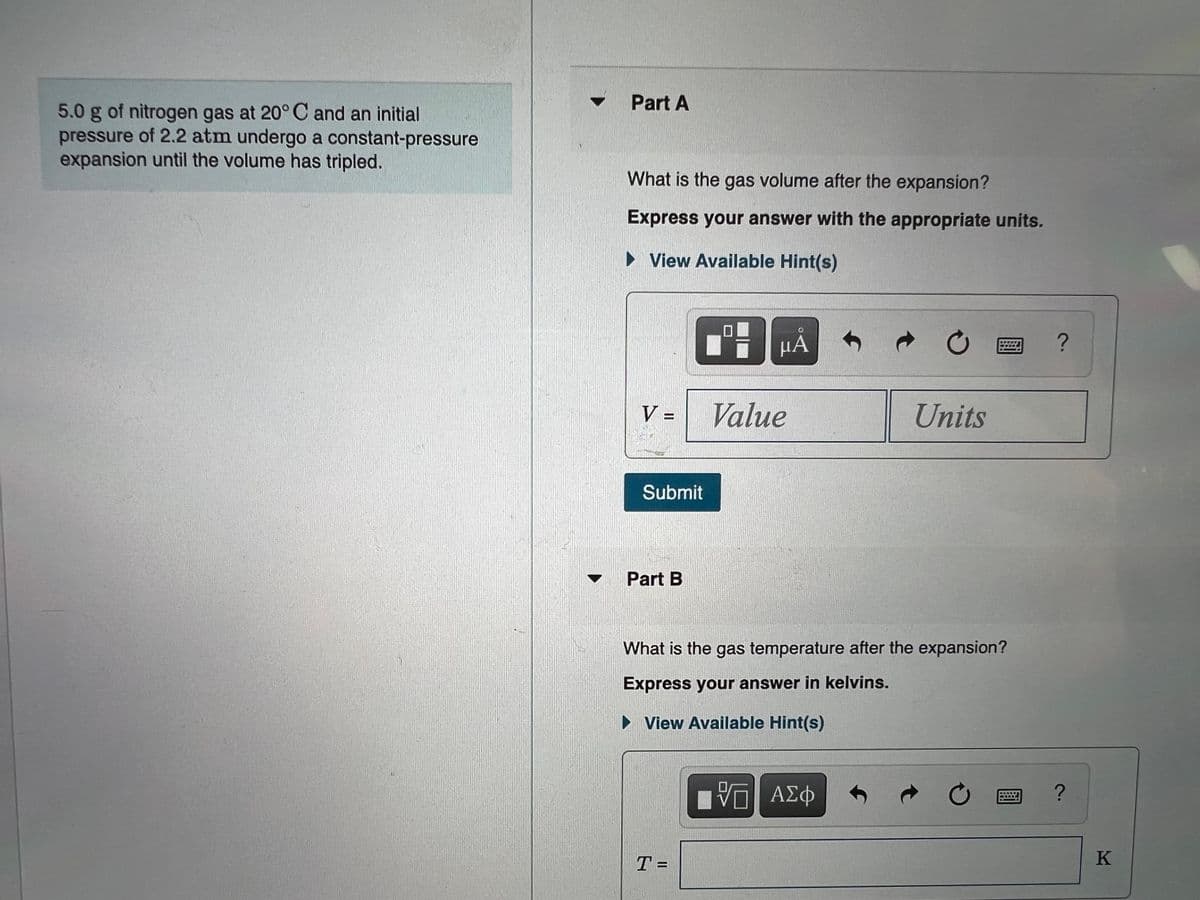
Transcribed Image Text:Part A
5.0 g of nitrogen gas at 20° C and an initial
pressure of 2.2 atm undergo a constant-pressure
expansion until the volume has tripled.
What is the gas volume after the expansion?
Express your answer with the appropriate units.
• View Available Hint(s)
µÀ
V =
Value
Units
Submit
▼
Part B
What is the gas temperature after the expansion?
Express your answer in kelvins.
• View Available Hint(s)
ΑΣφ
T =
K
Expert Solution
Step 1
Given,
The amount of Nitrogen gas (m)=5.0 g
Initial temperature of the gas
Initial Pressure of the Gas (P)=2.2 atm
Final volume of the gas=Triple of Initial volume of the gas
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, physics and related others by exploring similar questions and additional content below.Recommended textbooks for you

College Physics
Physics
ISBN:
9781938168000
Author:
Paul Peter Urone, Roger Hinrichs
Publisher:
OpenStax College


College Physics
Physics
ISBN:
9781938168000
Author:
Paul Peter Urone, Roger Hinrichs
Publisher:
OpenStax College
