Part A Calculate the mass percent composition of iron for Fe2 O3 (hematite). Express the mass percent to two decimal places. ΑΣφ ? % Part B Calculate the mass percent composition of iron for Fe3 O4 (magnetite). Express the mass percent to two decimal places. ? %
Part A Calculate the mass percent composition of iron for Fe2 O3 (hematite). Express the mass percent to two decimal places. ΑΣφ ? % Part B Calculate the mass percent composition of iron for Fe3 O4 (magnetite). Express the mass percent to two decimal places. ? %
Chemistry: An Atoms First Approach
2nd Edition
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Steven S. Zumdahl, Susan A. Zumdahl
Chapter5: Stoichiometry
Section: Chapter Questions
Problem 168CP
Related questions
Question
Please answer question 13 Part B, C, and D
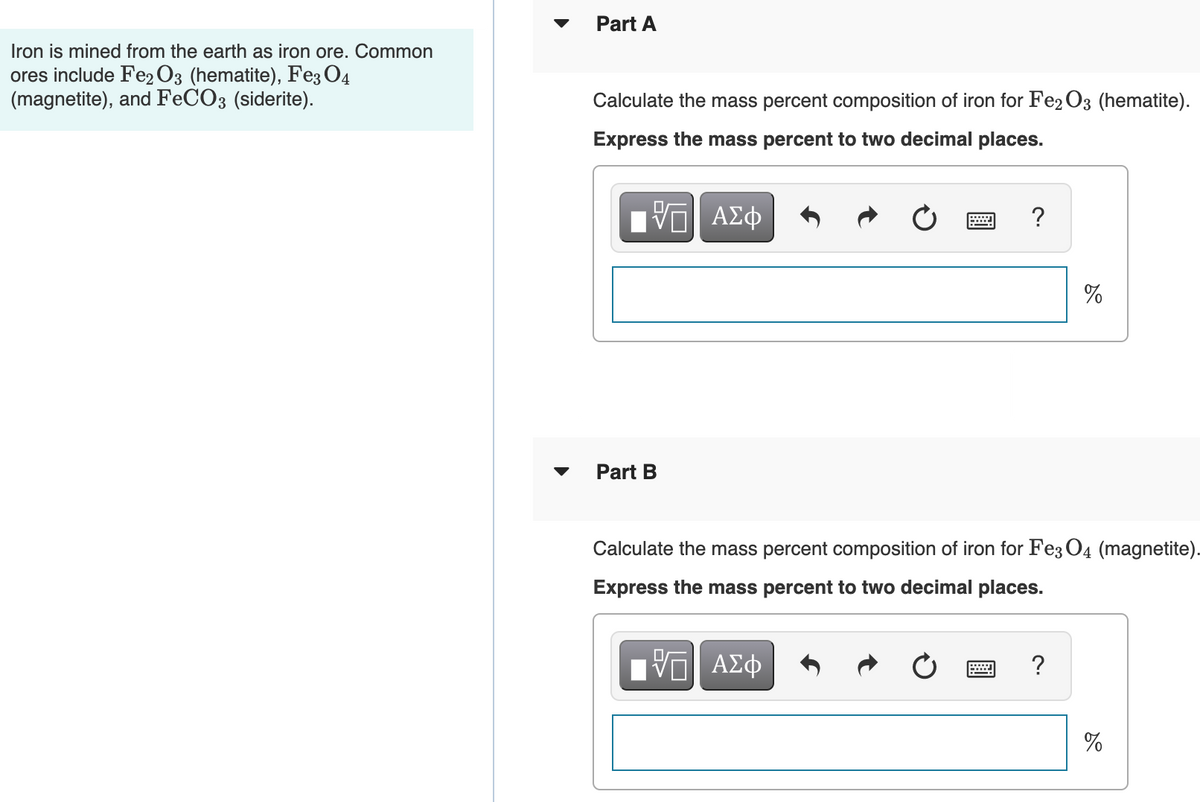
Transcribed Image Text:Part A
Iron is mined from the earth as iron ore. Common
ores include Fe2 O3 (hematite), Fe3 O4
(magnetite), and FECO3 (siderite).
Calculate the mass percent composition of iron for Fe2 O3 (hematite).
Express the mass percent to two decimal places.
Part B
Calculate the mass percent composition of iron for Fe3 O4 (magnetite).
Express the mass percent to two decimal places.
?
%
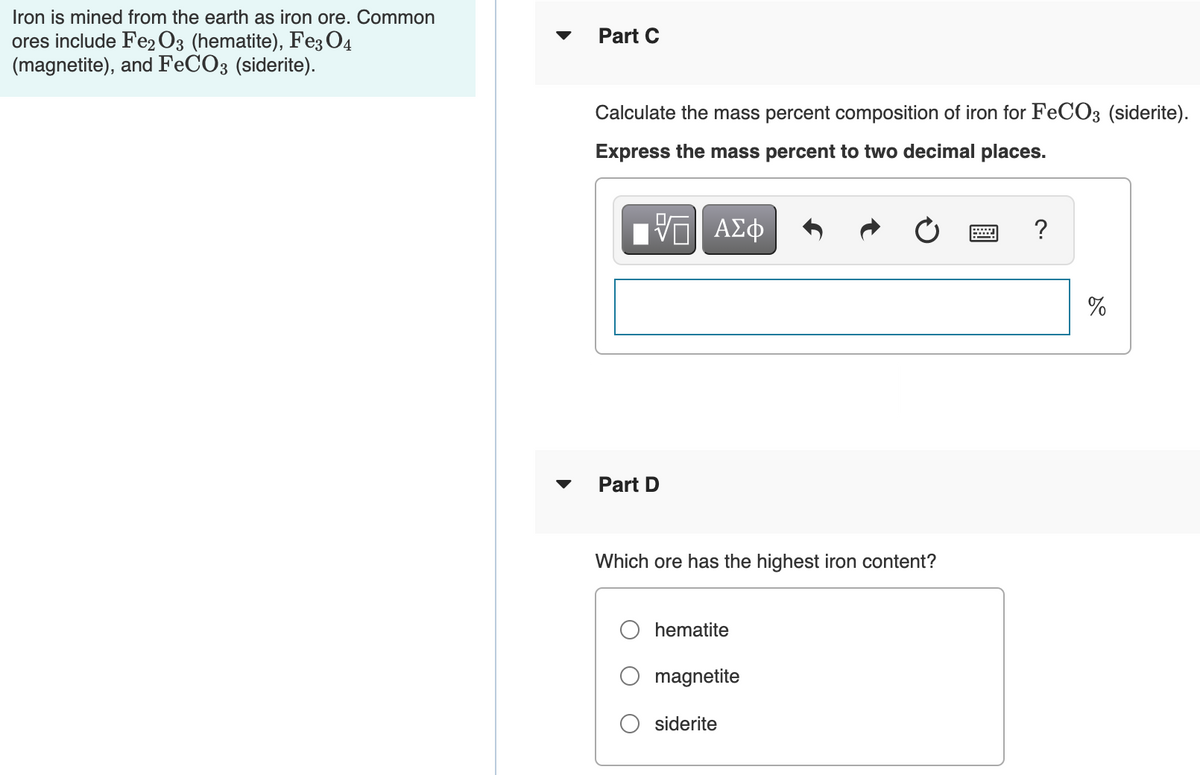
Transcribed Image Text:Iron is mined from the earth as iron ore. Common
Part C
ores include Fe2 O3 (hematite), Fe3 O4
(magnetite), and FECO3 (siderite).
Calculate the mass percent composition of iron for FeCO3 (siderite).
Express the mass percent to two decimal places.
?
%
Part D
Which ore has the highest iron content?
hematite
magnetite
siderite
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Recommended textbooks for you

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning