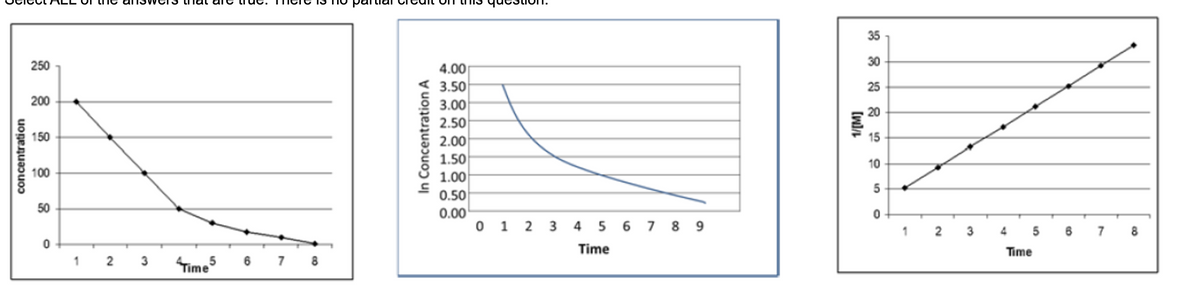
Part A: Find the value of the specific rate constant, k, for the data shown in the graphs in Question #1. Two data points from each graph are given below. First graph (time, conc): (4 s, 50 M) and (1 s, 200M) Middle graph (time, In(conc)): (5 s, 1.00) and (1 s, 3.50) Last graph (time, 1/conc): (6 s, 25 M-1) and (1 s, 5 M-1) Enter the value only in the box.
Part A: Find the value of the specific rate constant, k, for the data shown in the graphs in Question #1. Two data points from each graph are given below. First graph (time, conc): (4 s, 50 M) and (1 s, 200M) Middle graph (time, In(conc)): (5 s, 1.00) and (1 s, 3.50) Last graph (time, 1/conc): (6 s, 25 M-1) and (1 s, 5 M-1) Enter the value only in the box.
Chemistry & Chemical Reactivity
10th Edition
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Chapter14: Chemical Kinetics: The Rates Of Chemical Reactions
Section: Chapter Questions
Problem 45PS
Related questions
Question
Hello, could you please help me with this question. Also if you could please include the units for the specific rate constant k, in the the answer. Thank you!

Transcribed Image Text:QUESTION 2
Part A: Find the value of the specific rate constant, k, for the data shown in the graphs in Question #1. Two data points from each graph are given below.
First graph (time, conc): (4 s, 50 M) and (1 s, 200M)
Middle graph (time, In(conc)): (5 s, 1.00) and (1 s, 3.50)
Last graph (time, 1/conc): (6 s, 25 M-1) and (1 s, 5 M-1)
Enter the value only in the box.
Transcribed Image Text:35
250
30
4.00
< 3.50
3.00
2.50
2.00
1.50
1.00
0.50
0.00
0 1 2 3
25
200
20
150
15
10
100
50
4
5
6.
7 8 9
2
7
4
Time
Time
3
Times
7
concentration
1,
In Concentration A
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 2 images

Recommended textbooks for you

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning