Part A For the following pair, indicate which element has the lower first ionization energy: Match the words in the left column to the appropriate blanks in the sentences on the right. Make certain each sentence is complete before submitting your answer. Reset Help Ge Given the elements Cl and Ge. has the smaller first ionization energy. Ba Given the elements Pb and Sb, has the smaller first ionization energy. CI Given the elements Li and Na, has the smaller first ionization energy Na Li Given the elements Ba and Ti has the smaller first ionization energy. Sb Ti Pb Submit Request Answer
Part A For the following pair, indicate which element has the lower first ionization energy: Match the words in the left column to the appropriate blanks in the sentences on the right. Make certain each sentence is complete before submitting your answer. Reset Help Ge Given the elements Cl and Ge. has the smaller first ionization energy. Ba Given the elements Pb and Sb, has the smaller first ionization energy. CI Given the elements Li and Na, has the smaller first ionization energy Na Li Given the elements Ba and Ti has the smaller first ionization energy. Sb Ti Pb Submit Request Answer
Introductory Chemistry: A Foundation
9th Edition
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Donald J. DeCoste
Chapter11: Modern Atomic Theory
Section: Chapter Questions
Problem 11ALQ: r Questions 11—13, you will need to consider ionizations beyond the first ionization energy. For...
Related questions
Question
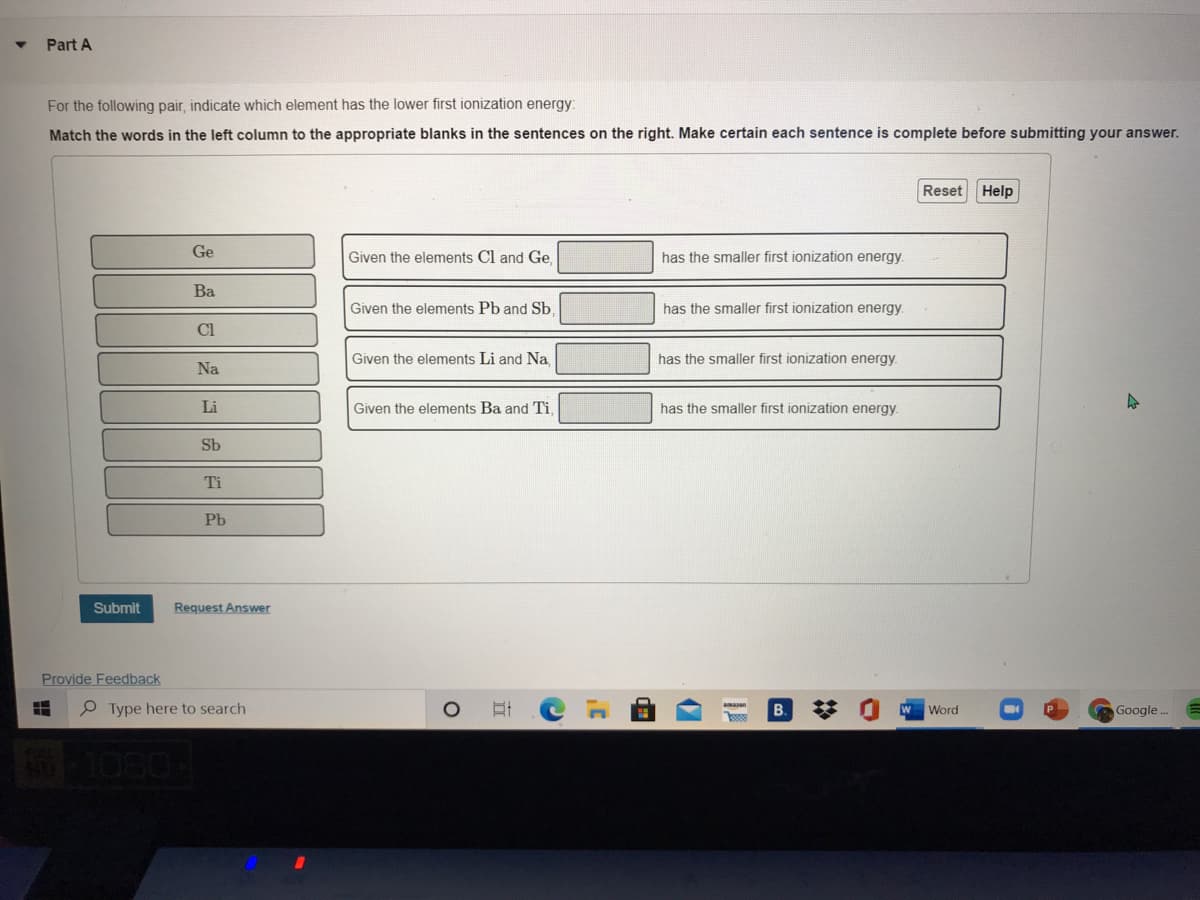
Transcribed Image Text:Part A
For the following pair, indicate which element has the lower first ionization energy:
Match the words in the left column to the appropriate blanks in the sentences on the right. Make certain each sentence is complete before submitting your answer.
Reset
Help
Ge
Given the elements Cl and Ge.
has the smaller first ionization energy.
Ba
Given the elements Pb and Sb.
has the smaller first ionization energy
Cl
Given the elements Li and Na,
has the smaller first ionization energy.
Na
Li
Given the elements Ba and Ti,
has the smaller first ionization energy.
Sb
Ti
Pb
Submit
Request Answer
Provide Feedback
P Type here to search
B.
W
Word
Google .
-1080-
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning