Consider the following statements as they apply to quantum numbers. Decide if each statement is True or False False Electrons in an s atomic orbital are the least shielded by electrons in other orbitals from the nuclear charge. False 2, only two orbitals are allowed, ones and one p Electrons in a multi-electron atom are placed in orbitals in order of increasing energy If m The secondary quantum number I indicates the shape of the orbital. If n True 0, the value of I must equal 0. False True Incorrect. Tries 9/99 Previous Tries Submit Answer This discussion is closed. Send Feedback
Consider the following statements as they apply to quantum numbers. Decide if each statement is True or False False Electrons in an s atomic orbital are the least shielded by electrons in other orbitals from the nuclear charge. False 2, only two orbitals are allowed, ones and one p Electrons in a multi-electron atom are placed in orbitals in order of increasing energy If m The secondary quantum number I indicates the shape of the orbital. If n True 0, the value of I must equal 0. False True Incorrect. Tries 9/99 Previous Tries Submit Answer This discussion is closed. Send Feedback
Principles of Modern Chemistry
8th Edition
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Chapter5: Quantum Mechanics And Atomic Structure
Section: Chapter Questions
Problem 55AP: The outermost electron in an alkali-metal atom is sometimes described as resembling an electron in...
Related questions
Question
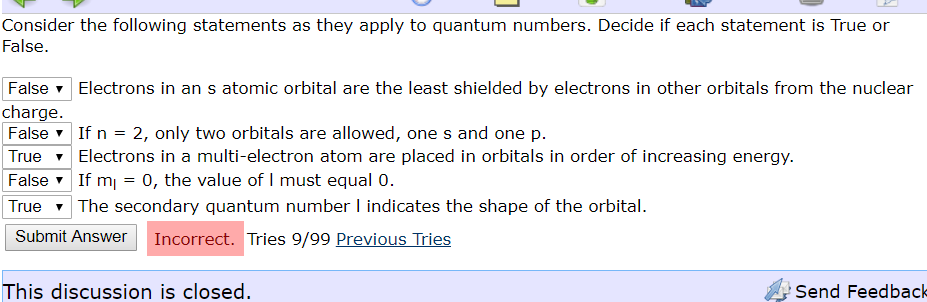
Transcribed Image Text:Consider the following statements as they apply to quantum numbers. Decide if each statement is True or
False
False Electrons in an s atomic orbital are the least shielded by electrons in other orbitals from the nuclear
charge.
False
2, only two orbitals are allowed, ones and one p
Electrons in a multi-electron atom are placed in orbitals in order of increasing energy
If m
The secondary quantum number I indicates the shape of the orbital.
If n
True
0, the value of I must equal 0.
False
True
Incorrect. Tries 9/99 Previous Tries
Submit Answer
This discussion is closed.
Send Feedback
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Recommended textbooks for you

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning