▼ ▼ Part A In order understand how this equation derived and why it holds true, the product f each side of the equation should be examined. What value and unit do you get when you multiply a concentration of 0.687 M by a volume of 0.500 L ? This question can be expressed as 0.500 L 0.687 mol X = T Express your answer to three significant figures with the appropriate units. ▸ View Available Hint(s) μA 1 C ? M₁V₁= Value Units Submit Part B When you need to produce a variety of diluted solutions of a solute, you can dilute a series of stock solutions. A stock solution has a significantly higher concentration of the given solute (typically 10¹ to 10¹ times higher than those of the diluted solutions). The high concentration allows many diluted solutions to be prepared using minimal amounts of the stock solution. What volume of a 6.61 M stock solution do you need to prepare 500. mL of a 0.0579 M solution of MgSO4? Express the volume to three significant figures with the appropriate units. ► View Available Hint(s) Di V₁ = Units Submit Value
▼ ▼ Part A In order understand how this equation derived and why it holds true, the product f each side of the equation should be examined. What value and unit do you get when you multiply a concentration of 0.687 M by a volume of 0.500 L ? This question can be expressed as 0.500 L 0.687 mol X = T Express your answer to three significant figures with the appropriate units. ▸ View Available Hint(s) μA 1 C ? M₁V₁= Value Units Submit Part B When you need to produce a variety of diluted solutions of a solute, you can dilute a series of stock solutions. A stock solution has a significantly higher concentration of the given solute (typically 10¹ to 10¹ times higher than those of the diluted solutions). The high concentration allows many diluted solutions to be prepared using minimal amounts of the stock solution. What volume of a 6.61 M stock solution do you need to prepare 500. mL of a 0.0579 M solution of MgSO4? Express the volume to three significant figures with the appropriate units. ► View Available Hint(s) Di V₁ = Units Submit Value
Chemical Principles in the Laboratory
11th Edition
ISBN:9781305264434
Author:Emil Slowinski, Wayne C. Wolsey, Robert Rossi
Publisher:Emil Slowinski, Wayne C. Wolsey, Robert Rossi
Chapter29: Synthesis And Analysis Of A Coordination Compound
Section: Chapter Questions
Problem 1ASA
Related questions
Question
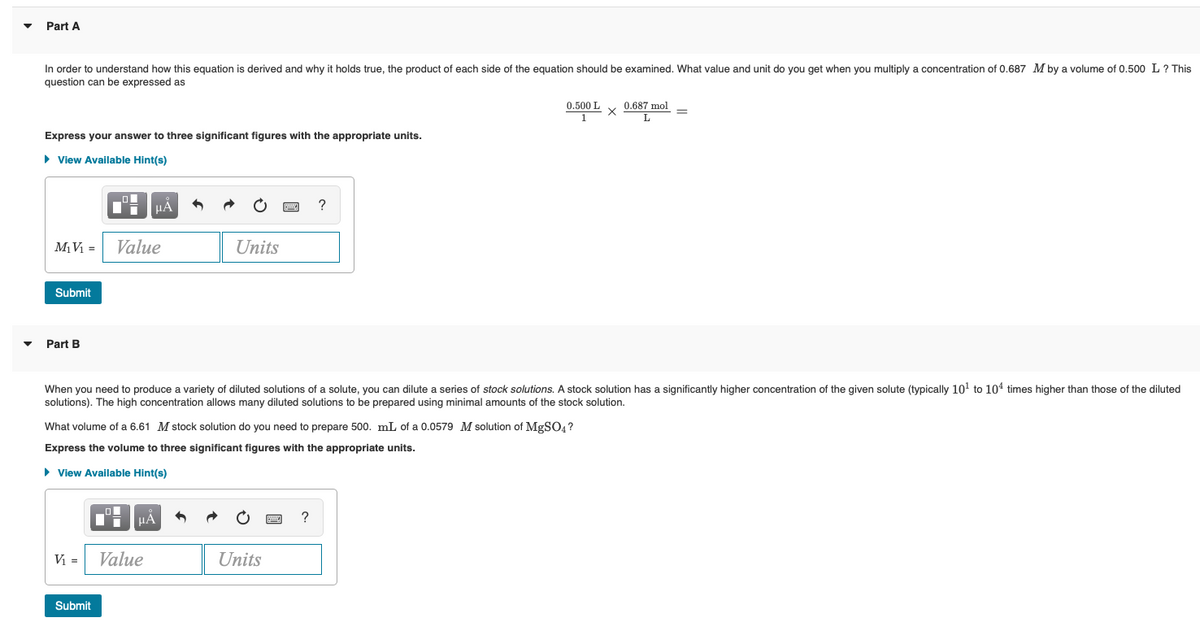
Transcribed Image Text:Part A
In order to understand how this equation is derived and why it holds true, the product of each side of the equation should be examined. What value and unit do you get when you multiply a concentration of 0.687 M by a volume of 0.500 L ? This
question can be expressed as
0.500 L
1
0.687 mol
X
L
Express your answer to three significant figures with the appropriate units.
► View Available Hint(s)
O
μÀ
?
M₁ V₁ = Value
Units
Submit
Part B
When you need to produce a variety of diluted solutions of a solute, you can dilute a series of stock solutions. A stock solution has a significantly higher concentration of the given solute (typically 10¹ to 104 times higher than those of the diluted
solutions). The high concentration allows many diluted solutions to be prepared using minimal amounts of the stock solution.
What volume of a 6.61 M stock solution do you need to prepare 500. mL of a 0.0579 M solution of MgSO4?
Express the volume to three significant figures with the appropriate units.
► View Available Hint(s)
μA
?
V₁ =
Value
Units
Submit
■
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 4 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemical Principles in the Laboratory
Chemistry
ISBN:
9781305264434
Author:
Emil Slowinski, Wayne C. Wolsey, Robert Rossi
Publisher:
Brooks Cole

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemical Principles in the Laboratory
Chemistry
ISBN:
9781305264434
Author:
Emil Slowinski, Wayne C. Wolsey, Robert Rossi
Publisher:
Brooks Cole

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


