The first step in the catabolism of most amino acids is the removal of the nitrogen atom by transfer to an a-keto acid, a reaction catalyzed by an enzyme called a transaminase. The a-keto acid acceptor is often a-ketoglutarate. Modify the structures in the product to show the products of the transamination of cysteine. Be sure to show functional groups with the charge and number of attached hydrogen atoms appropriate for pH 7.4. transaminase + O=C H₂N-CH + CH₂ CH₂ CH₂ SH Incorrect H₂N || CH | CH₂ | CH₂ I || O || n | CH₂ T SH
The first step in the catabolism of most amino acids is the removal of the nitrogen atom by transfer to an a-keto acid, a reaction catalyzed by an enzyme called a transaminase. The a-keto acid acceptor is often a-ketoglutarate. Modify the structures in the product to show the products of the transamination of cysteine. Be sure to show functional groups with the charge and number of attached hydrogen atoms appropriate for pH 7.4. transaminase + O=C H₂N-CH + CH₂ CH₂ CH₂ SH Incorrect H₂N || CH | CH₂ | CH₂ I || O || n | CH₂ T SH
Chapter29: The Organic Chemistry Of Metabolic Pathways
Section29.SE: Something Extra
Problem 30MP
Related questions
Question
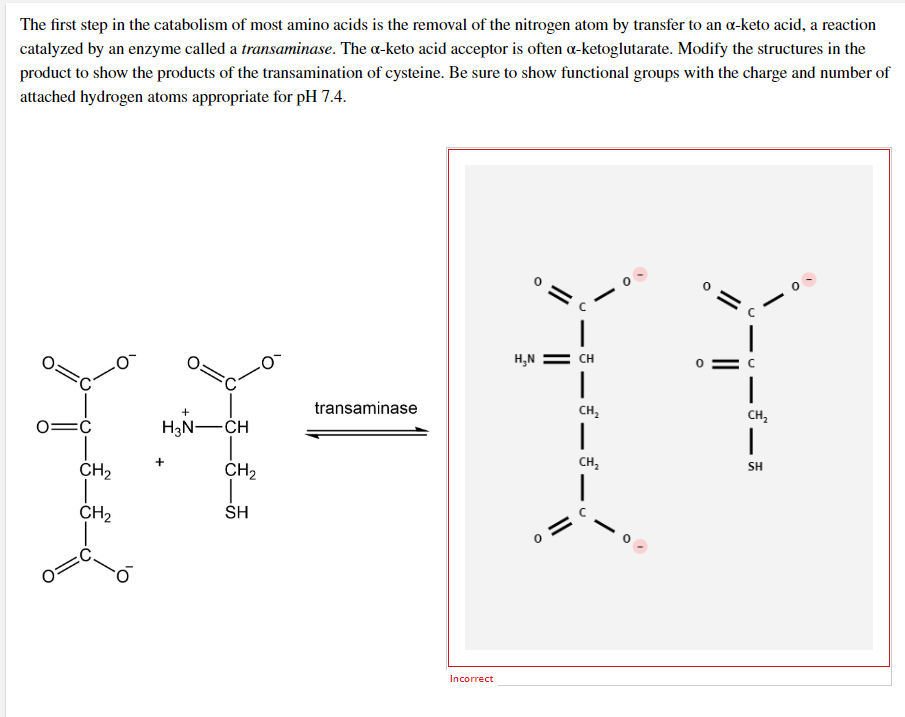
Transcribed Image Text:The first step in the catabolism of most amino acids is the removal of the nitrogen atom by transfer to an a-keto acid, a reaction
catalyzed by an enzyme called a transaminase. The a-keto acid acceptor is often a-ketoglutarate. Modify the structures in the
product to show the products of the transamination of cysteine. Be sure to show functional groups with the charge and number of
attached hydrogen atoms appropriate for pH 7.4.
transaminase
+
O=C
H₂N-CH
+
CH₂
CH₂
CH₂
SH
Incorrect
H₂N
||
CH
|
CH₂
|
CH₂
I
||
O
||
n
|
CH₂
T
SH
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning


Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning