Part A: Label the number of valence electrons each group on the periodic table has. Group 1 Group 2 Group 13 Group 14 Group 15 Group 16 Group 17 Group 18 What about Helium?
Part A: Label the number of valence electrons each group on the periodic table has. Group 1 Group 2 Group 13 Group 14 Group 15 Group 16 Group 17 Group 18 What about Helium?
General, Organic, and Biological Chemistry
7th Edition
ISBN:9781285853918
Author:H. Stephen Stoker
Publisher:H. Stephen Stoker
Chapter4: Chemical Bonding: The Ionic Bond Model
Section: Chapter Questions
Problem 4.6EP
Related questions
Concept explainers
Atomic Structure
The basic structure of an atom is defined as the component-level of atomic structure of an atom. Precisely speaking an atom consists of three major subatomic particles which are protons, neutrons, and electrons. Many theories have been stated for explaining the structure of an atom.
Shape of the D Orbital
Shapes of orbitals are an approximate representation of boundaries in space for finding electrons occupied in that respective orbital. D orbitals are known to have a clover leaf shape or dumbbell inside where electrons can be found.
Question
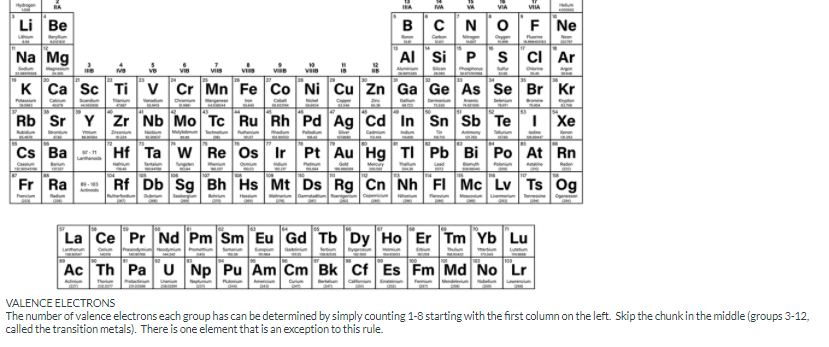
Part A:
Label the number of valence electrons each group on the periodic table has.
Group 1
Group 2
Group 13
Group 14
Group 15
Group 16
Group 17
Group 18
What about Helium?
Transcribed Image Text:IRA
MA
VIA
VIA
Li Be
BCNOF Ne
Na Mg
Al si Ps cI Ar
vie
viis
K Ca Sc Ti v Cr Mn Fe Co Ni Cu Zn Ga Ge As Se Br Kr
C
Rb SrY Zr Nb Mo Tc Ru Rh Pd Ag cd "In Sn Sb TeI Xe
Cs Ba
Hf Ta w Re os Ir Pt Au Hg TI Pb Bi Po At Rn
"Fr Ra Rf Db Sg Bh Hs Mt Ds Rg Cn Nh FI Mc Lv Ts Og
La Ce Pr Nd Pm Sm Eu Gd Tb Dy HoEr Tm Yb Lu
Ac Th Pa U Np Pu Am Cm Bk Cf Es Fm Md No Lr
VALENCE ELECTRONS
The number of valence electrons each group has can be determined by simply counting 1-8 starting with the first column on the left. Skip the chunk in the middle (groups 3-12,
called the transition metals). There is one element that is an exception to this rule.
Transcribed Image Text:Label the number of valence electrons each group on the periodic table has.
Group 1
|
4 valence electrons
Group 2
1 valence electron
Group 13
7 valence electrons
2 valence electrons
Group 14
6 valence electrons
3 valence electrons
5 valence electrons
Group 15
Group 16
8 valence electrons
Group 17
Group 18
What about Helium?
>
>
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

General, Organic, and Biological Chemistry
Chemistry
ISBN:
9781285853918
Author:
H. Stephen Stoker
Publisher:
Cengage Learning

Chemical Principles in the Laboratory
Chemistry
ISBN:
9781305264434
Author:
Emil Slowinski, Wayne C. Wolsey, Robert Rossi
Publisher:
Brooks Cole

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

General, Organic, and Biological Chemistry
Chemistry
ISBN:
9781285853918
Author:
H. Stephen Stoker
Publisher:
Cengage Learning

Chemical Principles in the Laboratory
Chemistry
ISBN:
9781305264434
Author:
Emil Slowinski, Wayne C. Wolsey, Robert Rossi
Publisher:
Brooks Cole

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781285199030
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Living By Chemistry: First Edition Textbook
Chemistry
ISBN:
9781559539418
Author:
Angelica Stacy
Publisher:
MAC HIGHER

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning