Part A MISSED THIS? Read Section 19.9 (Eages. ): Watch WE.19.1. Consider the following reaction Calculate AG for this reaction at 25 "Cunder the following condtions CH,OH(R) CO(g) + 2H, (R) Pen,on = 0.800 atm - 0.115 atm - 0.160 atm Pto (Note that AGOm -162.3 kJ/mol and AG,coe --137.2 kJ/mol.) Express your answer in kilojoules to three significant figures. AG= kJ
Part A MISSED THIS? Read Section 19.9 (Eages. ): Watch WE.19.1. Consider the following reaction Calculate AG for this reaction at 25 "Cunder the following condtions CH,OH(R) CO(g) + 2H, (R) Pen,on = 0.800 atm - 0.115 atm - 0.160 atm Pto (Note that AGOm -162.3 kJ/mol and AG,coe --137.2 kJ/mol.) Express your answer in kilojoules to three significant figures. AG= kJ
Chapter12: Gravimetric Methods Of Analysis
Section: Chapter Questions
Problem 12.20QAP
Related questions
Question
4
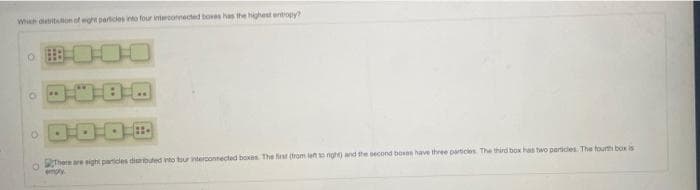
Transcribed Image Text:Wheh aletiton of eght particies into four intercornected boes has the highest entiopy
There are eight particles diubuted into tour intercontected boxes The first (from leno nigh) and the oecond boses have three particles The third box has two perices The fourth box is
empy
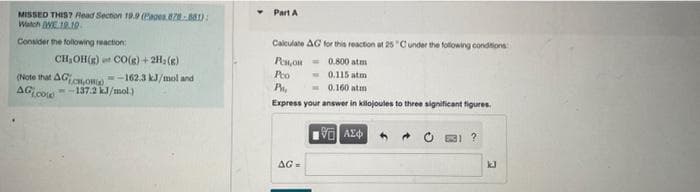
Transcribed Image Text:Part A
MISSED THIS? Read Section 19. (Eages. ):
Watch WE.19.1.
Consider the following reaction
Calculate AG for this reaction at 25 "Cunder the following condtions
CH,OH(R) CO(g) + 2H, (R)
Pen,on = 0.800 atm
- 0.115 atm
- 0.160 atım
Pto
(Note that AG,Om -162.3 kJ/mol and
AG co
--137.2 kJ/mol.)
Express your answer in kilojoules to three significant figures.
AG=
kJ
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning