Part A onsider the titration of a 31.0 mL sample of 0.125 mol L-1 H5COOH (K₂ = 6.3 x 10-5) with 0.105 mol L-1 aOH. Determine each quantity: the initial pH Express the pH to two decimal places. pH = ΜΕ ΑΣΦ Submit Request Answer Part B C. the volume of added base required to reach the equivalence point Express the volume in millilitres to three significant figures. V = V ΑΣΦ Submit Request Answer ? mL
Part A onsider the titration of a 31.0 mL sample of 0.125 mol L-1 H5COOH (K₂ = 6.3 x 10-5) with 0.105 mol L-1 aOH. Determine each quantity: the initial pH Express the pH to two decimal places. pH = ΜΕ ΑΣΦ Submit Request Answer Part B C. the volume of added base required to reach the equivalence point Express the volume in millilitres to three significant figures. V = V ΑΣΦ Submit Request Answer ? mL
Chemistry: Principles and Reactions
8th Edition
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:William L. Masterton, Cecile N. Hurley
Chapter14: Equilibria In Acid-base Solutions
Section: Chapter Questions
Problem 74QAP: Fifty cm3 of 1.000 M nitrous acid is titrated with 0.850 M NaOH. What is the pH of the solution (a)...
Related questions
Question
Nitesh
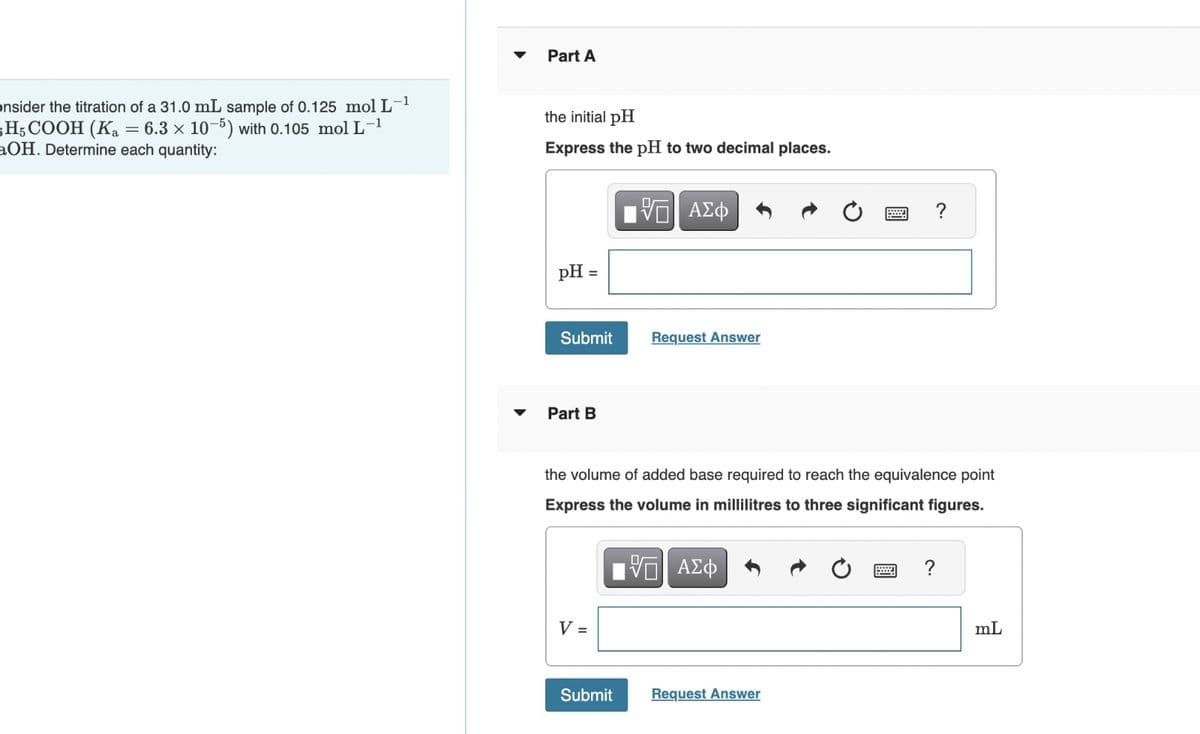
Transcribed Image Text:Part A
onsider the titration of a 31.0 mL sample of 0.125 mol L-1
H5COOH (K₂ = 6.3 x 10-5) with 0.105 mol L-1
aOH. Determine each quantity:
the initial pH
Express the pH to two decimal places.
pH =
ΜΕ ΑΣΦ
Submit
Request Answer
Part B
C.
the volume of added base required to reach the equivalence point
Express the volume in millilitres to three significant figures.
V =
V
ΑΣΦ
Submit
Request Answer
?
mL
AI-Generated Solution
Unlock instant AI solutions
Tap the button
to generate a solution
Recommended textbooks for you

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning