Part A - Reactions of Carbon Dioxide Gas 1. Place a small lump of dry ice into a large test-tube. To the dry ice add about 5 mL of water. After the gas has bubbled through the water for a few minutes (so that it may be assumed the solution is saturated with the gas), test the acidity of the solution by adding 1 drop of universal indicator solution and check the pH on the colour chart provided. Record your observations onto your results page. 2. Place two large test tubes in the test tube rack. In one tube place a small lump of dry ice and cover the mouth of the tube with a loose plug of cotton wool. Stand the test tube in a beaker of containing a little warm water (from the hot water tap) for a minute. The test tube will fill with carbon dioxide gas. In the meantime, obtain two small (100 or 150 mL) beakers, and place 20 mL of water in each and add 3 drops of universal indicator to cach. 3. Do this step quickly to avoid loss of CO2(g) from the test tube. Remove the cotton wool and tilt the test tube to allow the dry ice to slide into the second test tube and invert the first tube into one of the small beakers Co,(g) CO,(g) containing water (see adjacent figure). Transfer the cotton wool plug to the second test tube which should now have the solid CO, in it. 4. Warm the second tube in warm water for a minute, then remove the cotton wool, let the CO,(s) slide out into an empty beaker and invert the tube into the second small beaker. Add 20 mL of 2 M NAOH to the water in the second beaker and carefully move the test tube around to stir the solution (see adjacent figure). H,O(1) NaOH(aq) 5. Observe both test tubes over a 10 minute period, noting any changes in the liquid level in the two test tubes. Start part B in the meantime, but don't forget to record your observations from this part. RESULTS (Remember to not include spectator ions in ionic equations.) Part A - Reactions of Carbon Dioxide Gas | Observations at end of step 1.
Part A - Reactions of Carbon Dioxide Gas 1. Place a small lump of dry ice into a large test-tube. To the dry ice add about 5 mL of water. After the gas has bubbled through the water for a few minutes (so that it may be assumed the solution is saturated with the gas), test the acidity of the solution by adding 1 drop of universal indicator solution and check the pH on the colour chart provided. Record your observations onto your results page. 2. Place two large test tubes in the test tube rack. In one tube place a small lump of dry ice and cover the mouth of the tube with a loose plug of cotton wool. Stand the test tube in a beaker of containing a little warm water (from the hot water tap) for a minute. The test tube will fill with carbon dioxide gas. In the meantime, obtain two small (100 or 150 mL) beakers, and place 20 mL of water in each and add 3 drops of universal indicator to cach. 3. Do this step quickly to avoid loss of CO2(g) from the test tube. Remove the cotton wool and tilt the test tube to allow the dry ice to slide into the second test tube and invert the first tube into one of the small beakers Co,(g) CO,(g) containing water (see adjacent figure). Transfer the cotton wool plug to the second test tube which should now have the solid CO, in it. 4. Warm the second tube in warm water for a minute, then remove the cotton wool, let the CO,(s) slide out into an empty beaker and invert the tube into the second small beaker. Add 20 mL of 2 M NAOH to the water in the second beaker and carefully move the test tube around to stir the solution (see adjacent figure). H,O(1) NaOH(aq) 5. Observe both test tubes over a 10 minute period, noting any changes in the liquid level in the two test tubes. Start part B in the meantime, but don't forget to record your observations from this part. RESULTS (Remember to not include spectator ions in ionic equations.) Part A - Reactions of Carbon Dioxide Gas | Observations at end of step 1.
Introduction to General, Organic and Biochemistry
11th Edition
ISBN:9781285869759
Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Chapter22: Proteins
Section: Chapter Questions
Problem 22.62P: 22-62 Distinguish between intermolecular and intramolecular hydrogen bonding between backbone...
Related questions
Question
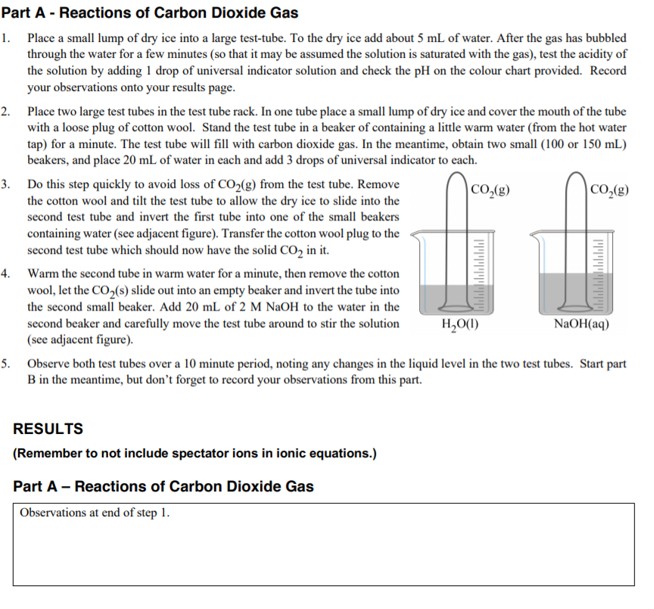
Transcribed Image Text:Part A - Reactions of Carbon Dioxide Gas
1. Place a small lump of dry ice into a large test-tube. To the dry ice add about 5 mL of water. After the gas has bubbled
through the water for a few minutes (so that it may be assumed the solution is saturated with the gas), test the acidity of
the solution by adding 1 drop of universal indicator solution and check the pH on the colour chart provided. Record
your observations onto your results page.
2. Place two large test tubes in the test tube rack. In one tube place a small lump of dry ice and cover the mouth of the tube
with a loose plug of cotton wool. Stand the test tube in a beaker of containing a little warm water (from the hot water
tap) for a minute. The test tube will fill with carbon dioxide gas. In the meantime, obtain two small (100 or 150 mL)
beakers, and place 20 mL of water in each and add 3 drops of universal indicator to each.
3. Do this step quickly to avoid loss of CO,(g) from the test tube. Remove
the cotton wool and tilt the test tube to allow the dry ice to slide into the
second test tube and invert the first tube into one of the small beakers
CO,(g)
|CO,(g)
containing water (see adjacent figure). Transfer the cotton wool plug to the
second test tube which should now have the solid CO, in it.
4. Warm the second tube in warm water for a minute, then remove the cotton
wool, let the CO,(s) slide out into an empty beaker and invert the tube into
the second small beaker. Add 20 mL of 2 M NaOH to the water in the
second beaker and carefully move the test tube around to stir the solution
(see adjacent figure).
H,O(1)
NaOH(aq)
5. Observe both test tubes over a 10 minute period, noting any changes in the liquid level in the two test tubes. Start part
B in the meantime, but don't forget to record your observations from this part.
RESULTS
(Remember to not include spectator ions in ionic equations.)
Part A - Reactions of Carbon Dioxide Gas
Observations at end of step 1.
ויויויויויויןיי
וו ןוייויויןיוין
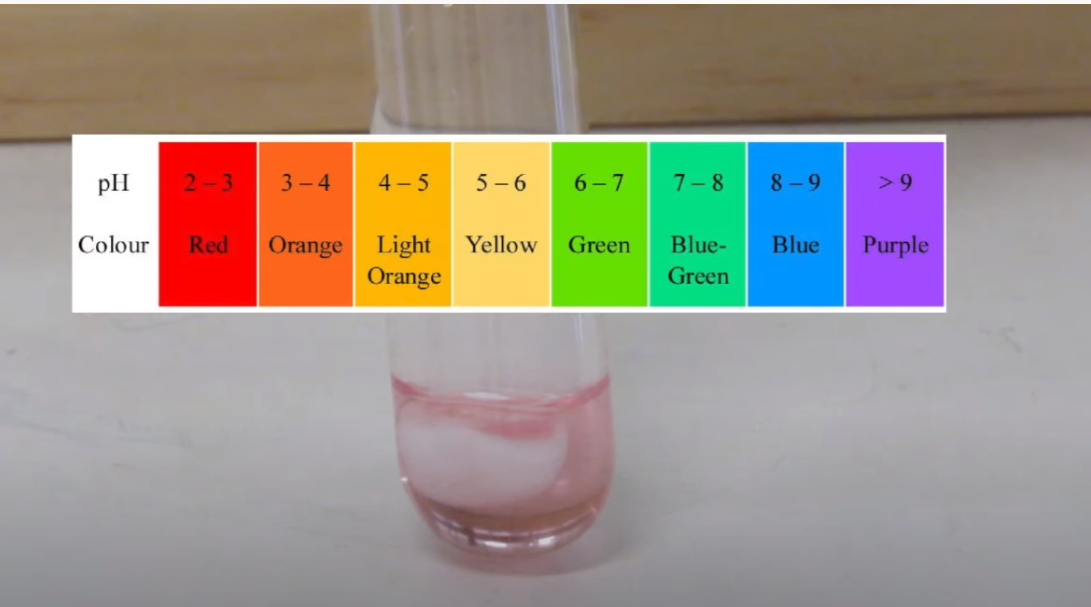
Transcribed Image Text:pH
3-4
4 – 5
5 – 6
6-7
7- 8
8 -9
>9
Orange Light
Orange
Colour
Red
Yellow
Green
Blue-
Blue
Purple
Green
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning