Disinfectant cleaner was the only substance that was entirely aqua Disinfectant Orange Juice Substance Red Coke Vinegar cleaner Cabbage Dark purple Dark orange Dark pink Dark pink Aqua Colour Notes and observations on the neutralisation experiment: When stirring the baking soda into the vinegar substance, smalls bubbles began to appear along with making fizzing sounds The substance immediately changed back to the original dark purple colour
Disinfectant cleaner was the only substance that was entirely aqua Disinfectant Orange Juice Substance Red Coke Vinegar cleaner Cabbage Dark purple Dark orange Dark pink Dark pink Aqua Colour Notes and observations on the neutralisation experiment: When stirring the baking soda into the vinegar substance, smalls bubbles began to appear along with making fizzing sounds The substance immediately changed back to the original dark purple colour
Principles of Instrumental Analysis
7th Edition
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
ChapterA1: Evaluation Of Analytical Data
Section: Chapter Questions
Problem A1.22QAP
Related questions
Question
Describe the results from the neutralisation experiment. Use your knowledge of chemistry to explain the neutralisation of an acid with a base.
(*Note- results are provided in the photo)
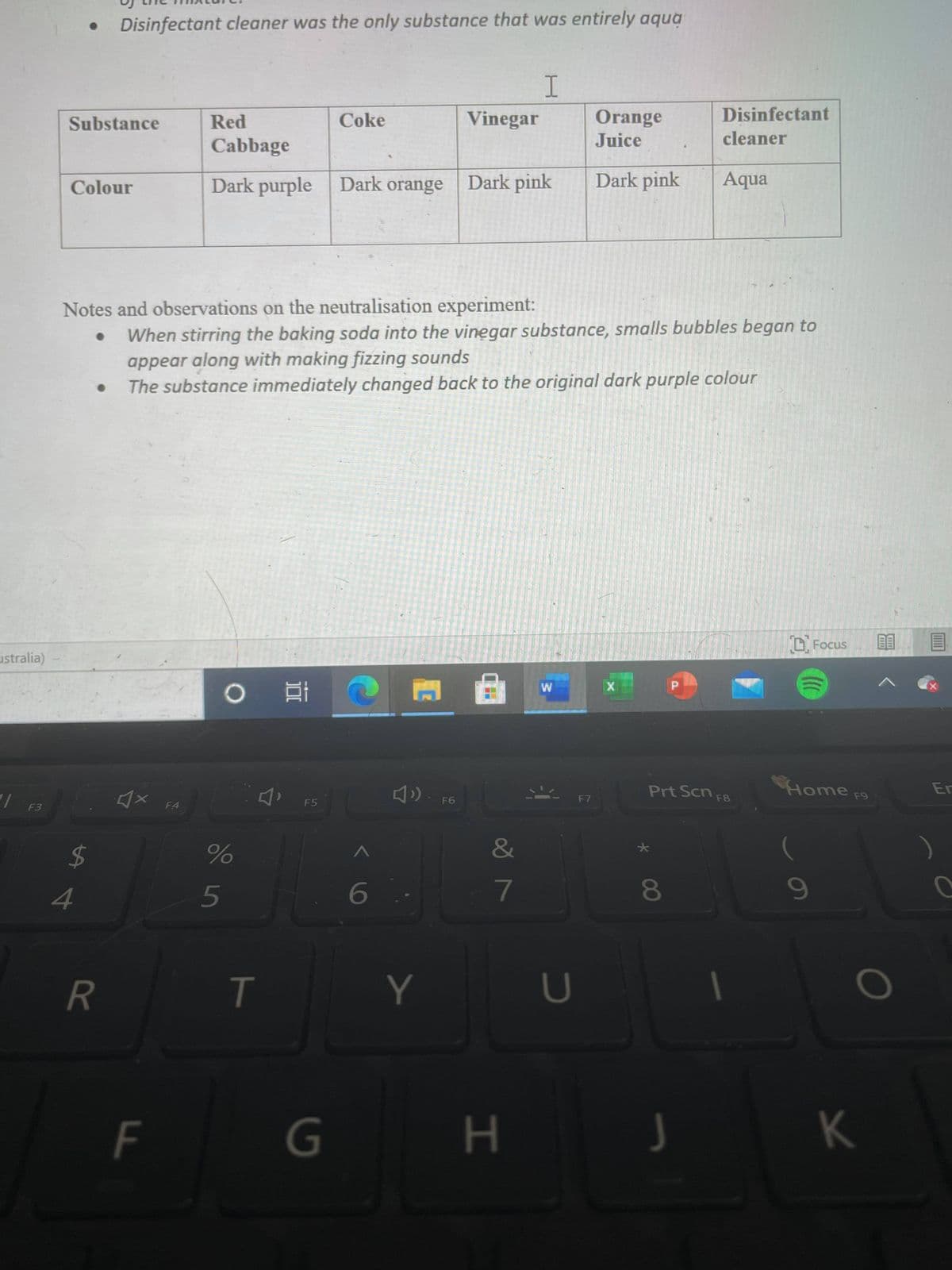
Transcribed Image Text:Disinfectant cleaner was the only substance that was entirely aqua
Substance
Red
Coke
Vinegar
Orange
Disinfectant
Cabbage
Juice
cleaner
Colour
Dark purple Dark orange
Dark pink
Dark pink
Aqua
Notes and observations on the neutralisation experiment:
When stirring the baking soda into the vinegar substance, smalls bubbles began to
appear along with making fizzing sounds
The substance immediately changed back to the original dark purple colour
O Focus
ustralia)
). F6
Prt Scn Fg
Home ro
Er
F7
F3
F4
F5
%24
&
6
81
T
Y U
F
G H
K
4.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning


EBK A SMALL SCALE APPROACH TO ORGANIC L
Chemistry
ISBN:
9781305446021
Author:
Lampman
Publisher:
CENGAGE LEARNING - CONSIGNMENT

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning


EBK A SMALL SCALE APPROACH TO ORGANIC L
Chemistry
ISBN:
9781305446021
Author:
Lampman
Publisher:
CENGAGE LEARNING - CONSIGNMENT

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning
