Part A The propane fuel (C, H) used in gas barbeques burns according to the thermochemical C,Hs(9) + 50:(9) + 3CO,(G) + 4H,0(9) A,H = -2217 kJ mol Ifa pork roast must absorb 1.7 x 10 kJ to fully cook, and if only 10 % of the heat produced by the barbeque is actually absorbed by the roast, what mass of COz is emitted into the atmosphere during the griling of the pork roast? Express your answer using two significant figures. bole undo do reset keyboard shortcuts help Templaes SyAYA mco, = Submit Regust Answt
Part A The propane fuel (C, H) used in gas barbeques burns according to the thermochemical C,Hs(9) + 50:(9) + 3CO,(G) + 4H,0(9) A,H = -2217 kJ mol Ifa pork roast must absorb 1.7 x 10 kJ to fully cook, and if only 10 % of the heat produced by the barbeque is actually absorbed by the roast, what mass of COz is emitted into the atmosphere during the griling of the pork roast? Express your answer using two significant figures. bole undo do reset keyboard shortcuts help Templaes SyAYA mco, = Submit Regust Answt
Chemistry: Principles and Practice
3rd Edition
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Chapter5: Thermochemistry
Section: Chapter Questions
Problem 5.86QE: One of the components of jet engine fuel is n-dodecane, C12H26(), which has a standard enthalpy of...
Related questions
Question
please help
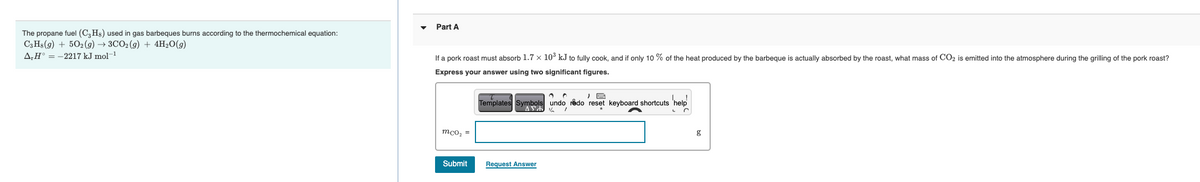
Transcribed Image Text:Part A
The propane fuel (C,Hs) used in gas barbeques burns according to the thermochemical equation:
СзHа (9) + 502(9) —> ЗСО2(9) + 4H20(9)
A,H° = -2217 kJ mol-1
If a pork roast must absorb 1.7 x 10° kJ to fully cook, and if only 10 % of the heat produced by the barbeque is actually absorbed by the roast, what mass of CO2 is emitted into the atmosphere during the grilling of the pork roast?
Express your answer using two significant figures.
Templates Symbols undo rådo reset keyboard shortcuts 'help
mco, =
g
Submit
Request Answer
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning